b Collaborative Innovation Center of Chemical Science and Engineering(Tianjin), Tianjin 300072, China
As high production-volume (HPV) chemicals in industry,esters have been used extensively for the production of paints,inks, adhesives,polyesters,drugs,agrochemicals,flavoring agents and fragrances [1]. Esters are generally prepared by the acylation of alcohols with acyl halides,anhydrides,carboxylic acids,esters,etc. In addition,the reactions of carboxylic acids with diazomethane, alkyl halides,other electrophiles such as tert-butyl acetoacetate, dimethyl sulfate and methyl trichloroacetate are also common esterification methods [1, 2]. Among these methods,acylation with anhydrides is facile and widely used in organic synthesis and manufacturing of pharmaceuticals [1]. Various catalysts,such as poly(N-vinylimidazole),p-toluenesulfonyl chloride,V(HSO4)3 and immobilized cobalt(Ⅱ),have been employed for acylation with anhydrides [3, 4, 5, 6].
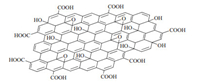
Graphite oxide (GO) has attracted much attention recently because its exfoliated single layered structure (graphene oxide) has been intensively exploited in nanomaterials as a precursor of graphene (or reduced graphene oxide) [7]. GO is generally prepared by treating graphite with strongly oxidative reagents, such as KMnO4 and KClO3 [8, 9]. It is an amorphous,nonstoichiometric,hygroscopic,moderately toxic and weakly acidic solid. The precise structure of GO is ambiguous up to now. It is generally thought that the continuous aromatic lattice of graphite is interrupted by various oxygen-containing functional groups,such as hydroxyls,carbonyls,epoxides and carboxyls,and all these groups are introduced to the surface or the edge of the basal plane of graphene oxide under the harsh oxidative conditions. One of the proposed models for the structure of graphene oxide is shown in Scheme 1 [9].
|
Download:
|
| Scheme. 1. Simplified structure model of graphene oxide. | |
Recently,GO has emerged as efficient catalysts in various organic transformations [10, 11]. As a carbocatalyst for redox reactions,it catalyzed the oxidation of alcohols by air [12],the selective oxidation of thiols to disulfides and sulfides to sulfoxides [13],the one-pot base-free synthesis of amides from aromatic aldehydes and secondary amines [14]. As an acid catalyst,GO could catalyze the room temperature ring opening of epoxides with alcohols [15],the one-pot conversion of carbohydrates into 5- ethoxymethylfurfural [16],the production of bio-additives from glycerol esterification [17],aza-Michael addition of amines to activated alkenes [18],the condensation of 4-hydroxycoumarin and aryl glyoxals in the synthesis of new dicoumarols [19]. Moreover,Loh et al. explored a GO-catalyzed carbon-carbon or/and carbon-heteroatom bond formation strategy to functionalize primary amines in tandem to produce a series of valuable products [20]. We also disclosed that GO could serve as a catalyst for esterification and transesterification [21],tetrahydropyranylation/ depyranylation of alcohols and phenols [22],and the deprotection of Boc-protected alcohols and phenols [23]. GO could act as a weak solid acid to protonate the carbonyls in carboxylic acids or esters and facilitate the nucleophilic substitution with alcohols. As a continuation of our research work,we herein wish to report that acetic anhydride can be activated by GO and react with alcohols and phenols to yield the corresponding acetates efficeiently.
2. ExperimentalReagents and apparatus: Graphite powder (synthetic,99.99%) was purchased from Tainjin Huabei Reagent Co.,of China. KMnO4, NaNO3,98% H2SO4,36%-38% HCl and 30% H2O2 were received as guaranteed reagents and used without further purification. All the other reagents were received as analytical reagents and used without further purification. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were performed on a Bruker Avance Ⅲ (400 MHz) spectrometer using CDCl3 as a solvent and TMS as an internal standard,chemical shifts were given in ppm. FT-IR spectroscopy was performed using a Bruker ALPHA spectrophotometer. Powder XRD was performed on a BDX-3300 X-ray diffractometer at 40 kV and 20 mA with 2θ ranging from 108 to 408 using Cu Ka radiation (λ = 0.15418 nm). XPS spectrum was recorded using an XPS-PHI5000VersaProbe and AES-PHI670xi Scanning Auger Nanoprobe instrument with a monochromated Al Ka radiation (hv = 1486.6 eV).
Preparation of GO: A 250 mL three-necked round-bottom flask equipped with a mechanical stirrer was charged with graphite powder (3.0 g),NaNO3 (1.5 g) in an ice bath. Concentrated H2SO4 (69 mL) was added slowly into the mixture. Then KMnO4 (9.0 g) was added in portions under stirring to prevent the temperature from exceeding 20 ℃. The reaction mixture was stirred at 35 ℃ for 7 h. After being cooled to room temperature,additional KMnO4 (9.0 g) was added slowly in portions into the mixture,then the reaction mixture was stirred at 35 ℃ for 12 h. After the flask was cooled to room temperature,the resulted suspension was poured into 420 mL of ice-cold deionized water and 3 mL of 30% H2O2 was added. Then the mixture was filtered and the isolated solid was washed with 200 mL of 30% HCl,200 mL of deionized water and filtered in succession five times. Then washing with water and filtering was repeated five times until the pH value of the filtrate was 6-7. The solid was dried in a desiccator in the presence of P2O5 under vacuum until its weight was constant to afford GO as a dark brown powder.
General procedure for GO-catalyzed acetylation of alcohols and phenols: To a solution of alcohols or phenols (3 mmol) and Ac2O (4.5 mmol; 9 mmol for dihydroxybenzenes) in CH3CN or CH2Cl2 (2 mL) in a round-bottom flask was added GO (5 wt% of alcohols or phenols). The mixture was stirred at 20 ℃ and the progress of the reaction was monitored by thin layer chromatography (TLC) analysis. After the completion of the reaction,the catalyst was filtered and washed with ethyl acetate (20 mL × 2). The filtrate was combined and washed with 15 mL of saturated sodium carbonate. The organic phase was dried over Na2SO4 and evaporated in vacuo to obtain the desired products.
3. Results and discussionGO was prepared by the improved Hummers method with a combination of KMnO4,NaNO3 and H2SO4 [24]. As-prepared GO was characterized by IR,XRD and XPS. IR spectra showed the peaks of the oxygen-containing functional groups (Fig. S1 in Supporting information,3400 cm-1 for O-H,1739 cm-1 for C=O,1227 cm-1 for epoxy C-O,1056 cm-1 for C-O). XRD pattern revealed a broad peak centered at 11.46,which is characteristic for GO (Fig. S2 in Supporting information). XPS analysis indicated that a large amount of oxygen-containing functional groups were incorporated during the harsh oxidation process (Fig. S3 in Supporting information).
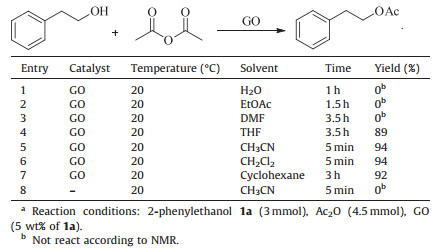
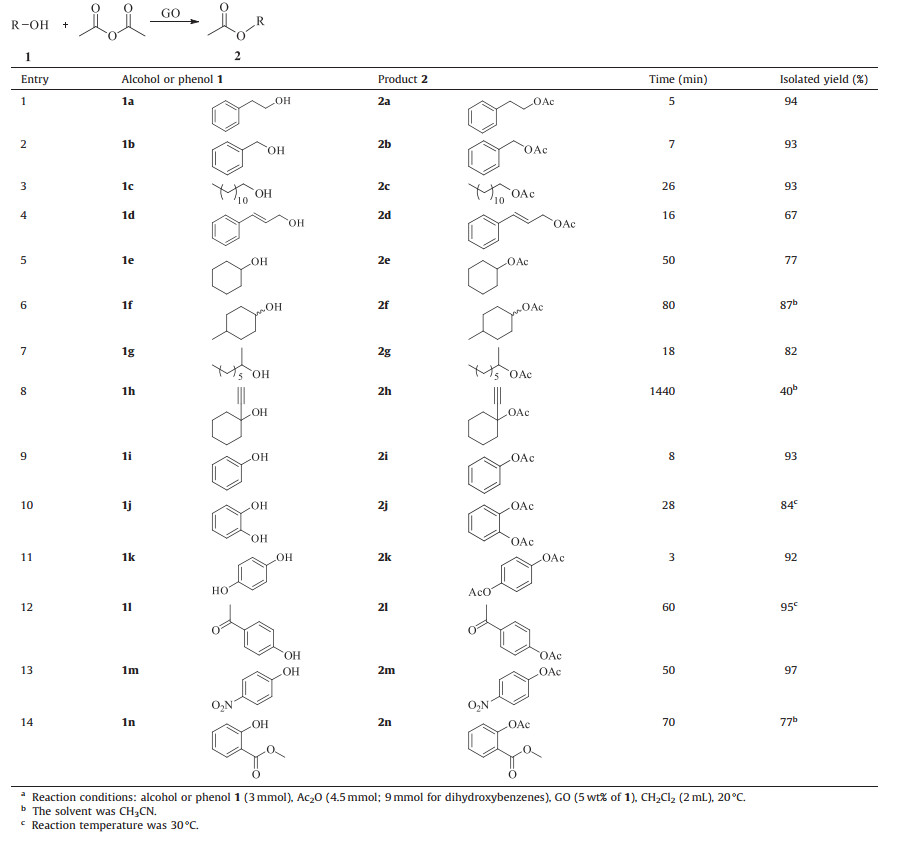
We then examined the reaction of alcohols with acetic anhydride in the presence of GO. First,we tried several solvents for the acetylation of 2-phenylethanol and found that acetonitrile or dichloromethane was best; we could produce the corresponding ester in a yield of 94% (Table 1). Thus acetonitrile or dichloromethane was used as the solvent for all reactions. As shown in Table 2,the reaction of 2-phenylethanol with acetic anhydride was accelerated by GO remarkably. The reaction was completed in 5 min (entry 1,Table 2),while the product was hardly detected by 1H NMR in the reaction without GO (entry 8,Table 1). Other primary alcohols,such as benzyl alcohol and 1-dodecanol,were also converted to their acetates in excellent yields under similar conditions (entries 2 and 3,Table 2). In the case of cinnamic alcohol,2d was obtained in a yield of 67%. Some undesired side reactions occurred. We attempted to identify the by-products,but they were difficult to isolate by column chromatography (entry 4, Table 2). When the hydroxy groups in the substrates are more sterically hindered,such as cyclohexanol,4-methylcyclohexanol and 2-octanol,the reaction was relatively slow. It needed longer reaction time (50 min,80 min and 18 min) to convert these alcohols completely,but good yields of 77%,87% and 82% still could be achieved,respectively (entries 5,6 and 7,Table 2). In the case of tertiary alcohol 1-ethynyl-1-cyclohexanol,the reaction seemed more difficult and 1-ethynyl-1-cyclohexanol was acetylated in a yield of 40% in 1440 min (entry 8,Table 2). Phenol was efficiently transformed to its acetate in short reaction time (entry 9,Table 2). As for substituted phenols with electron donating or electron withdrawing groups,such as catechol,hydroquinone,p-acetylphenol,and p-nitrophenol (entries 10 to 13,Table 2),the corresponding acetates were prepared in excellent yields. Even a sterically hindered phenol could be converted to its acetate in a yield of 77% in 70 min (entry 14,Table 2). When GO was used as the catalyst,it needed 4.5 mmol of acetic anhydride to react with 3 mmol of alcohols or phenols. While other catalysts,such as p-toluenesulfonyl chloride [4] and heterogeneous cobalt(Ⅱ) Salen [6], no less than 5 mmol of acetic anhydride was used to react with 1 mmol of alcohols or phenols. Besides,our reaction temperature was 20 ℃,lower than the 50 ℃ that cobalt(Ⅱ) Salen-catalyzed reactions needed [6].
|
|
Table 1 Optimization of the reaction conditionsa |
|
|
Table 2 GO-catalyzed acetylation of alcohols and phenols with Ac2Oa |
Because the residual K,Mn in the GO may also have catalytic ability,we used the AAS to detect the content of Mn and K,and their content proved to be about 400 ppm. Further experiments showed that either Mn or K had little effect on the acetylation reaction (Table S1). Moreover,we have evaluated the reusability of GO. In the first cycle,yield of 94% was achieved in 5 min. While in the second cycle,a satisfied yield of 80% was obtained by extending the reaction time to 50 min. In the third cycle,only a yield of 38% was obtained even though the reaction time was prolonged to 300 min,which might be caused by the possible partial loss of the carboxyl groups in GO.
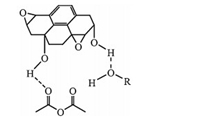
Although there have been different explanations about the origin of GO’s acidity in water [7, 9, 25],it is generally thought that the acidity mainly comes from the carboxyls on the edges of GO sheets in organic solvents. After being stirred overnight,a 0.1 g/L aqueous GO dispersion gave a pH value of 4.6. Accordingly,the catalytic mechanism of GO is illustrated as shown in Scheme 2 (GO is represented by coronene-1-carboxylic acid for the sake of simplicity). GO can protonate the carbonyl in acetic anhydride and make it more reactive toward the nucleophilic attack of alcohols or phenols. Then the acetate forms with the release of proton to regenerate GO’s structure through proton transfer. Furthermore, there are abundant oxygen-containing groups,especially hydroxy groups,on the surface of GO. These hydroxy groups tend to form hydrogen bonds with both acetic anhydride and alcohols or phenols (Scheme 3) [8]. Thus,these reactants are accumulated on the surface of GO,which also promote the reaction between them. Phenols are very reactive,which is probably due to the p-p interactions between the benzene ring of phenol and the sp2- conjugated domains in the GO structure.

|
Download:
|
| Scheme. 2. Plausible reaction mechanism of GO-catalyzed acetylation. | |
|
Download:
|
| Scheme. 3. Plausible hydrogen bonds between GO, acetic anhydride and alcohols/phenols. | |
4. Conclusion
In summary,we demonstrated a GO-catalyzed acetylation of various alcohols and phenols. GO showed highly catalytic activity and the acetates of primary and secondary alcohols were obtained in high yields in short reactiontime undermild conditions. Phenols were so reactive that electron deficient phenols could also be converted to their acetates in excellent yields. As a solid acid catalyst for these reactions,GO is easily available,cheap, moderately toxic,weakly acidic and environmentally benign. Since the acetyl group is an important protecting group of hydroxy group,the GO-catalyzed acetylation of alcohols and phenols with acetic anhydride may provide a mild,facile and eco-friendly method for the protection of alcohols and phenols.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2016.01.005.
| [1] | P.G.M. Wuts, T.W. Greene. Greene's Protective Groups in Organic Synthesis, fourth ed., John Wiley and Sons. New Jersey (2007) |
| [2] | J. Otera, J. Nishikido, Esterification:Methods Reactions and Applications, second ed., WILEY-VCH Verlag GmbH & Co, Weinheim, 2010. |
| [3] | N.G. Khaligh. Poly(N-vinylimidazole) as an efficient catalyst for acetylation of alcohols, phenols, thiols and amines under solvent-free conditions. RSC Adv. 3 (2013) 99–110 |
| [4] | A. Khazaei, A. Rostami, F.Mantashlo. p-Toluenesulfonyl chloride as a new and effective catalyst for acetylation and formylation of hydroxyl compounds under mild conditions. Chin. Chem. Lett. 21 (2010) 1430–1434 |
| [5] | F. Shirini, A.R. Sakhaei, M. Abedini. V(HSO4)3 catalyzed chemoselectivity acetylation of alcohols and phenols in solution and under solvent-free conditions. Chin. Chem. Lett. 20 (2009) 439–443 |
| [6] | F. Rajabi. A heterogeneous cobalt(Ⅱ) Salen complex as an efficient and reusable catalyst for acetylation of alcohols and phenols. Tetrahedron Lett. 50 (2009) 395–397 |
| [7] | D. Chen, H.B. Feng, J.H. Li. Graphene oxide:preparation, functionalization, and electrochemical applications. Chem. Rev. 112 (2012) 6027–6053 |
| [8] | D.R. Dreyer, S. Park, C.W. Bielawski, R.S. Ruoff. The chemistry of graphene oxide. Chem. Soc. Rev. 39 (2010) 228–240 |
| [9] | D.R. Dreyer, A.D. Todd, C.W. Bielawski. Harnessing the chemistry of graphene oxide. Chem. Soc. Rev. 43 (2014) 5288–5301 |
| [10] | C.K. Chua, M. Pumera. Carbocatalysis:the state of "metal-free" catalysis. Chem. Eur. J. 21 (2015) 12550–12562 |
| [11] | S. Navalon, A. Dhakshinamoorthy, M. Alvaro, H. Garcia. Carbocatalysis by graphene-based materials. Chem. Rev. 114 (2014) 6179–6212 |
| [12] | D.R. Dreyer, H.P. Jia, C.W. Bielawski. Graphene oxide:a convenient carbocatalyst for facilitating oxidation and hydration reactions. Angew. Chem. Int. Ed. 49 (2010) 6813–6816 |
| [13] | D.R. Dreyer, H.P. Jia, A.D. Todd, J.X. Geng, C.W. Bielawski. Graphite oxide:a selective and highly efficient oxidant of thiols and sulfides. Org. Biomol. Chem. 9 (2011) 7292–7295 |
| [14] | S. Kumari, A. Shekhar, H.P. Mungse, O.P. Khatri, D.D. Pathak. Metal-free one-pot synthesis of amides using graphene oxide as an efficient catalyst. RSC Adv. 4 (2014) 41690–41695 |
| [15] | A. Dhakshinamoorthy, M. Alvaro, P. Concepción, V. Forné s, H. Garcia. Graphene oxide as an acid catalyst for the room temperature ring opening of epoxides. Chem. Commun. 48 (2012) 5443–5445 |
| [16] | H.L. Wang, T.S. Deng, Y.X. Wang, et al. Graphene oxide as a facile acid catalyst for the one-pot conversion of carbohydrates into 5-ethoxymethylfurfural. Green Chem. 15 (2013) 2379–2383 |
| [17] | X.Q. Gao, S.H. Zhu, Y.W. Li. Graphene oxide as a facile solid acid catalyst for the production of bioadditives from glycerol esterification. Catal. Commun. 62 (2015) 48–51 |
| [18] | S. Verma, H.P. Mungse, N. Kumar, et al. Graphene oxide:an efficient and reusable carbocatalyst for aza-Michael addition of amines to activated alkenes. Chem. Commun. 47 (2011) 12673–12675 |
| [19] | S. Khodabakhshi, B. Karami, K. Eskandari, S. Jafar Hoseinia, A. Rashidib. Graphene oxide nanosheets promoted regioselective and green synthesis of new dicoumarols. RSC Adv. 4 (2014) 17891–17895 |
| [20] | C.L. Su, R. Tandiana, J. Balapanuru, et al. Tandem catalysis of amines using porous graphene oxide. J. Am. Chem. Soc. 137 (2015) 685–690 |
| [21] | J.M. Qi, Y.L. Xu, N. Ma, F.F. Sun. Graphite oxide-catalyzed esterification and transesterification. Chin. J. Org. Chem. 33 (2013) 1839–1846 |
| [22] | L. Xu, A.W. Yang, N. Ma. Graphite oxide as an efficient and reusable catalyst for tetrahydropyranylation/depyranylation of alcohols and phenols. Chin. J. Org. Chem. 33 (2013) 2004–2009 |
| [23] | Y.L. Xu, J.M. Qi, F.F. Sun, N. Ma. Carbocatalysis:reduced graphene oxide-catalyzed Boc protection of hydroxyls and graphite oxide-catalyzed deprotection. Tetrahedron Lett. 56 (2015) 2744–2748 |
| [24] | W.S. Hummers Jr., R.E. Offeman. Preparation of graphitic oxide. J. Am. Chem. Soc. 80 (1958) 1339 |
| [25] | A.M. Dimiev, L.B. Alemany, J.M. Tour. Graphene oxide Origin of acidity, its instability in water, and a new dynamic structural model. ACS Nano 7 (2013) 576–588 |
 2016, Vol. 27
2016, Vol. 27