b Department of Pharmacology, Technocrats Institute of Technology-Pharmacy, Anand Nagar, Bhopal, Madhya Pradesh 462021, India ;
c School of Pharmaceutical Sciences, Jaipur National University, Jaipur Rajasthan, India
The conventional multi-step preparation of a complex molecule generally involves a large number of synthetic operations, including extraction and purification processes in each individual step. This not only leads to synthetic inefficiency but also generates large amounts of waste [1]. Multi-component reactions (MCRs) can be distinguished from classical,sequential two-component synthetic processes in that they use three or more chemical starting materials as the input for product formation. When reaction involves three component it is referred as 3-MCR,if four it is 4-MCR and so on. Up to seven starting components have been used,and MCRs have often been shown to produce higher product yields than classical chemistry [2]. Multicomponent reactions (MCRs) are defined as the one-pot processes that combine at least three reactants to selectively form a single product containing essentially all the atoms of the reactants [3]. Ideally,all reaction equilibrium in the complex MCR mixture are reversible,and the last,the product-forming reaction step is irreversible,thus providing the driving force to shift all intermediates and starting materials towards a single final product.
MCRs allow the creation of several bonds in a single operation and offer remarkable advantages such as convergence,operational simplicity,facile automation,reduction in the number of workup/ extraction/purification steps,and hence minimize waste generation,rendering the transformations green. Therefore,the design of new green MCRs has attracted great attention,especially in the areas of drug discovery,organic synthesis,and material science [4]. Moreover,improving already known MCRs also is of substantial interest in current organic synthesis.
There are numerous methods available for the synthesis of 1-4 dihydropyridine (1-4 DHP) bearing polyhydroquinolines. A classical method involves the three component condensation of an aldehyde with ethylacetoacetate,and ammonia in acetic acid or refluxing alcohol [5]. Recently several new methodologies have been reported for the synthesis of polyhydroquinolines including the use of (YbOTf)3 [6],(ScOTf)3 [7],HClO4-SiO2 [8],L-proline [9], bakers yeast [10],organocatalyst [11],p-toluene-sulphonic acid [12],ZnO [13],Ni-nanoparticles [14],PPA-SiO2 [15],microwave [16],Cs2.5H0.5PW12O40 [17],FeF3 [18],TiO2 nanoparticles as catalysts [19]. These methods have their own merits and shortcomings.
Seizure is a clinical expression of abnormal neuron firings within the brain,occurs with or without the loss of consciousness leading to epilepsy. Approximately 0.4%-1% of the global population suffers from epilepsy. All the current antiepileptic drugs have dose-related toxicity and idiosyncratic side effects [20].
With a 1,4-dihydropyridine (1,4-DHP) parent nucleus,indenoquinoline derivatives have shown a diverse range of biological activities such as 5-HT receptor binding [21],antitumor activity [22, 23],cytotoxic activity [24],actylcholinesterase inhibition [25], antimalarial [26] anti-inflammatory activities [27],Topo I/II inhibition [28],DNA intercalation [29],antiploriferative [30], anti-mycobacterial [31],antihyperglycemic,and lipid modulating [32] activities. Owing to these diverse biological activities,these compounds have distinguished themselves as heterocycles of profound biological significance.
2. Experimental 2.1. ChemistryThe chemicals and reagents were purchased from various chemical companies such as Alfa-Aesar,HiMedia,Merck India and CDH. The progress of the reaction was monitored by thin layer chromatography (TLC- ethyl acetate: n-hexane-1:3) analysis using pre-coated silica gel G plates using UV chamber for visualization of TLC spots. IR spectra were recorded on a Shimadzu FT-IR spectrophotometer using KBr pellets. 1H NMR spectra were obtained using a Bruker’s AVANCE-III 400 MHz FT NMR spectrometers using CDCl3 as a solvent and TMS as an internal standard. The chemical shifts were expressed in ppm. Mass spectra were determined on an Applied Biosystems 3200 Q Trap LC/MS/MS instrument.
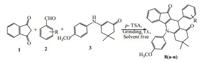
General experimental procedure for the synthesis of 5-(4- methoxyphenyl)-7,7-dimethyl-10-phenyl-7,8-dihydro-5H-indeno [1,2-b]quinoline-9,11(6H,10H)-dione 8(a-n): a mixture containing 1,3-indanedione 1 (1 mmol),aryl-aldehyde 2 (1 mmol),enaminone 3 (1 mmol) in the presence of a catalytic amount (10 mol%) of p-TSA and ethanol (1.0 mL) was thoroughly ground using a mortar and pestle of appropriate size till the completion of reaction as indicated by TLC analysis. The resulting red colored product was washed with water and was air dried to afford the crude product. The crude product was extracted by ethyl acetate. The organic layer was dried over anhydrous sodium sulphate and evaporated to dryness to give the crude product. The pure product was obtained by recrystallization from methanol (Scheme 1). The physical characterizations of the synthesized compounds are presented in Table 1.
|
Download:
|
| Scheme. 1. Synthetic route for target compounds 8(a-n). | |
|
|
Table 1 Physical characterization of the synthesized compounds 8(a-n). |
2.2. Biological activity
The anticonvulsant evaluation (MES) was carried out according to the phase Ⅰ tests of antiepileptic drug development program [33, 34]. Phenytoin (25 mg/kg) was dissolved in an aqueous Tween 80 (3% v/v,0.9% NaCl) solution. Negative control groups consisted of 3% Tween 80 solution. The tested compounds were prepared as suspensions in aqueous Tween 80 (3% v/v,0.9% NaCl),and injected intraperitoneally in a standard volume of 0.05 mL/20 g body weight.
The anticonvulsant activity of the titled compounds 8(a-n) were evaluated by the maximal electroshock seizure (MES) method. In the MES method seizures were elicited with a 60 Hz alternating current of 50 mA intensity in mice. The current was applied via corneal electrodes for 0.2 s. Protection against the spread of MES-induced seizures was defined as abolition of hind limb tonic extensor component of the seizure in half or more of the animals. After 30 min of administration,the anticonvulsant activity of compounds 8(a-n) was evaluated in the MES test. The data were analyzed by one-way ANOVA followed by the Dunnett’s test using the Graph Pad Prism program. All values were expressed as mean ± SEM. Comparison of anticonvulsant activity by the MES seizure method (compared with control) for compounds and the observations were taken.
The neurotoxicity of the compounds was measured in mice by the Rotarod test. The mice were trained to stay on an accelerating Rotarod of a diameter of 3.2 cm that rotates at 10 rpm. Trained mice were given an intraperitoneal injection of the test compounds. Neurotoxicity was indicated by the inability of the animal to maintain equilibrium on the rod for at least 1 min in each of the trials. The results are shown in Table 2. Statistical analysis of the results is expressed as mean SEM; n represents the number of animals. Data obtained from pharmacological experiments were analyzed by one way analysis of variance (ANOVA) followed by the Dunnet’s test using GraphPad Prism version 5.02. A p-value of less than 0.05 was considered statistically significant.
|
|
Table 2 Anticonvulsant activity and neurotoxicity screening of the synthesize compounds in maximal electroshock tests and Rotarod test. |
2.3. Docking study
The docking analysis of all molecules was performed using Maestro,version 9.0 implemented from Schro ¨dinger molecular modeling suite. The molecules were sketched in the 3D format using ‘‘build panel’’ and the LigPrep module was used to produce low-energy conformers. The crystal structures of serotonin 5-HT2A receptor (PDB ID: 2VT4) were retrieved from Protein Data Bank (www.rcsb.org). The protein was prepared by giving preliminary treatment such as adding hydrogen,adding missing residues, refining the loop with prime and finally minimized using OPLS- 2005 force field. Grid for molecular docking was generated with bound co-crystallized ligand. Molecules were docked using Glide in the standard precision mode,with up to three poses saved. Ligands were kept flexible by producing the ring conformations and by penalizing non-polar amide bond conformations,whereas the receptor was kept rigid throughout the docking studies. All other parameters of the Glide module were maintained at their default values. The lowest energy conformation was selected for the prediction of ligand interactions with the active sites of serotonin 5-HT2A receptor.
3. Results and discussion 3.1. ChemistryAll titled compounds 8(a-n) were synthesized using the synthetic protocols presented in Scheme 1. In an initial endeavor, 1 equiv. of 1,3 indanedione 1,benzaldehyde 2a,and enaminone 3 were refluxed in ethanol under catalyst free conditions. After 4 h, only 55% of the expected product 4a was obtained following workup and recrystallization from ethanol. In an attempt to improve the yield of the reaction and knowing the benefits of grinding,the same reaction was performed using an inexpensive p-TSA as catalyst under solvent-free conditions at room temperature. All the ingredients of the reaction were taken in a mortar, mixed thoroughly and ground well at room temperature to the stipulated time. It was observed that the mixture,which was initially in a partial liquid state,later solidified during the process of grinding to a brick red solid mass. Thin layer chromatography (TLC) indicated the complete conversion to the desired product. The crude product was extracted by ethyl acetate. The organic layer was dried over anhydrous sodium sulphate and evaporated to dryness to give 5-(4-methoxyphenyl)-7,7-dimethyl-10-phenyl- 7,8-dihydro-5H-indeno[1,2-b]quinoline-9,11(6H,10H)-dione 8a (80%). The pure product was obtained by recrystallization from methanol. A possible explanation for the improved yield in solvent-free conditions is that the eutectic mixture having uniform distribution of the reactants brings the reacting species in close proximity to react than in solvent.
A variety of substrates were subjected to this reaction condition and the desired products were obtained in good to excellent yields (Table 1). It is evident that electron rich and electron deficient aldehydes as well as heterocyclic aldehyde such as furfuraldehyde (2n),reacted smoothly to produce the desired products in high yields.
The purity of the synthesized compounds was checked by TLC and melting points were determined in open capillary tubes using a melting point apparatus and are uncorrected. The synthesized products were characterized by IR,1H NMR,Mass and elemental analysis. The IR spectrum of compounds showed one aromatic C-H stretching band around 3066 cm-1,four aromatic C=C stretching bands around 1630,1585,1510 and 1450 cm-1 and one aromatic C-H def around 887 cm-1 confirming the aromatic skeleton in the structure. Two aliphatic C-H stretching bands (CH3) were observed around 2960 cm-1 and 2930 cm-1 showing the presence of methyl group. Further all the derivatives showed a sharp characteristic C=O stretching band around 1680 cm-1 confirming the presence of cycloalkenone group and C-N stretching band around 1363 indicating the formation of quinoline ring. Derivatives 8b,8c,8d,and 8e showed absorption peak around 700 cm-1 due to C-Cl group. Derivatives 8f,8g,and 8i showed O-H stretching band around 3255 cm-1 and C-O stretching band around 1180 cm-1 indicating the presence of phenolic group. Derivatives 8h,8i,and 8l showed two characteristic absorption peaks around 1230 and 1030 cm-1 of C-O-C stretching band due to methoxy group. Derivative 8m showed a weak N-H stretching band at 3350 cm-1 due to NH2 group. Derivatives 8j and 8k showed absorption peaks around 1560 and 1395 cm-1 due to NO2 group. Moreover all the synthesized derivatives 8(a-n) showed two characteristic C-O-C stretching peaks around 1224 and 1030 cm-1,further confirming the presence of aromatic methoxy group. The 1H NMR spectrum of 8b shows two sharp singlets at δ 0.906 and 0.977 for the 3 protons of two methyl groups. Four proton multiplet of CH2 were recorded at d 2.008-2.149. A singlet of 3 protons at δ 3.944 corresponds to OCH3. A characteristic one proton singlet of CH was seen at δ 5.373. Eleven aromatic protons were observed in the range of δ 6.845-7.535 further confirming the structure of compound 8b. The molecular mass of the compound was confirmed by mass spectra (MS-EI m/z 496). Finally elemental analysis authenticates the results. Similarly a series of 5-(4-methoxyphenyl)-7,7-dimethyl- 10-phenyl-7,8-dihydro-5H-indeno[1,2-b]quinoline-9,11(6H,10H)- dione were synthesized and evaluated by spectral data.
3.2. Pharmacology 3.2.1. Determination of ED50Preliminary experiments were carried out in mice (n=6). Tested compounds were administered orally in different doses to identify the range of doses that cause zero and 100% mortality of animals. A range of doses was determined for each compound. In different groups,tested compounds were given i.p. in doses of 10, 20,25,30,40 and 50 mg/20 g of body weight. The ED50 was evaluated by the Pöch and Pancheva method [35]. The test compounds were administrated i.p. at different doses and animals were kept under observation for mortality as well as any behavioral changes for evaluation of a possible anticonvulsant effect.
3.2.2. Anticonvulsant activityIn general it is known that the maximal electroshock (MES) model,through an electrical stimulus,induces generalized tonic- clonic seizures. Therefore it is used to help identify those compounds that prevent seizure spread [36]. All the synthesized compounds were evaluated for their anticonvulsant activity using MES method using phenytoin as a standard drug along with their neurotoxicity effect and the data are summarized in Table 2. The MES activity model appears to be highly predictive against protecting generalized tonic-clonic seizures by anticonvulsants [37]. These seizures are very reproducible and are electro physiologically comparable with human seizures. Compounds that are found to be active in the MES seizure test are generally regarded to be significantly useful candidates in the treatment of partial generalized seizures.
Most of the synthesized derivatives 8(a-n) exhibited moderate to good anticonvulsant activity in the MES screening. Out of several tested compounds in the electroshock investigation,three derivatives 8b,8e,and 8k were found to be significantly active at a dose of 40 mg/kg (*** P < 0.001). These compounds contain electron withdrawing groups such as a chloro and a nitro group at the aryl ring attached to the indeno[1,2-b]quinoline nucleus. Compounds 8a,8c,8d,and 8j were also found to be active in the MES test (**P < 0.01). The in vivo data in mice confirmed the absorption of compounds from gastrointestinal tract and also their penetration into the central nervous system. The inhibition of electrically induced seizures that is characteristic for Phenytoin and Phenytoin drugs may imply the influence on the voltage dependent Na+ channels as the most plausible mechanism of anticonvulsant action.
On correlating the structures of the title compounds with their anticonvulsant activity,it has been observed that compounds containing electron withdrawing groups (8b,8e,and 8k) showed better activity than the compounds having electron releasing groups (8f,8g,and 8i). However,the non-substituted (8a) derivatives exhibit moderate anticonvulsant activity.
3.2.3. Neurotoxicity screening (NT)The neurotoxicity of the synthesized derivatives 8(a-n) was determined using the minimal motor impairment-Rotarod test. The Rotarod test indicates that all active compounds 8(a-n) showed the absence of neurotoxicity at the administered dose i.e., 40 mg/kg as compared to phenytoin,which also showed an absence of neurotoxicity at a dose level of 25 mg/kg.
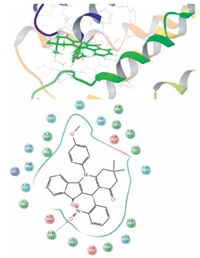
3.3. Docking analysisThe synthesized compound library was docked to a homology model of the serotonin 5-HT2A receptor using Glide docking algorithm and the binding affinity of the compounds were evaluated in terms of Glide score (Table 3). Fluoxetine was used as reference standard for the docking studies. The results of the docking studies revealed variable binding affinity of the synthesized compounds to the receptor with Glide score ranging from -7.216 (8f) to -4.789 (8i). The interaction of derivative 8k with 2VT4 is shown in Fig. 1. The key amino acid interactions observed are THR118,ASP121,VAL122,ASP200,VAL202,THR203,TYR207, ALA208,SER212,SER215,PHE306,PHE307,ASN310,ASN313, VAL314,ARG317,ASP322,PHE325,VAL326,TYR333,and ASN329. Further,a good correlation was observed between the experimental biological activities for derivatives 8(a-n). Similarly,compounds 8b,8e,and 8k with good docking scores also showed potent anticonvulsant activity.
|
Download:
|
| Fig. 1. Docking of 8k with 2VT4 and its two dimension interaction. | |
|
|
Table 3 Docking score of synthesized derivatives 8(a-n). |
However,derivative 8f had the highest affinity within the series but it showed insignificant anticonvulsant activity. The most probable reason might be presence of polar OH group that renders the compound hydrophilic and impeding its ability to cross the blood brain barrier. A similar phenomenon was also observed for compounds 8m having an NH2 substitution and 8g having a p-OH group substitution. Both the compounds showed weak anticonvulsant activity despite having a good docking score. Overall,the results of the molecular docking studies of synthesized derivatives revealed very good binding interactions with the receptor and also support the experimental biological activity shown by these compounds. These results also vindicate the application of docking studies prior to synthesis and biochemical testing of new analogs in efforts to discover potential anticonvulsant agents.
4. ConclusionIn summary,we have developed a simple and efficient multicomponent domino synthesis of indeno[1,2-b]quinoline derivatives using the reaction of 1,3-indanedione,aryl-aldehyde, enaminone and p-toluene sulphonic acid (10 mol%) as an efficient catalyst at ambient temperature using the grinding technique. The workup procedure is simple and excludes costly column chromatography procedure. This method gives high yielding of pure products in short reaction time. The use of green,nontoxic, economical catalyst p- toluene sulphonic acid has rendered this method eco-friendly.
Further,all the synthesized derivatives were evaluated for their anticonvulsant activity using the maximal electroshock (MES) method using phenytoin as a standard drug along with their neurotoxicity effect. Derivatives 8b,8e,and 8k exhibited significant anticonvulsant activity (P < 0.001). The neurotoxicity study clearly revealed that all the tested compounds are non-toxic at 40 mg/kg and so,new compounds can be considered as good templates for further developmental studies and this study may lead to further investigations aimed at discovering new drugs for the treatment of anticonvulsant disorders. The molecular modeling studies also predicted good binding interactions of most active molecules with the serotonin 5-HT2A receptor (2VT4). Therefore,it can be safely concluded that synthesized derivatives 8(a-n) would represent a useful model for further investigation in the development of a new class of anticonvulsant agents.
| [1] | Y. Gu. Multicomponent reactions in unconventional solvents:state of the art. Green Chem. 14 (2012) 2091–2128 |
| [2] | L. Weber. Multi-component reactions and evolutionary chemistry. Drug Dis. Today 7 (2002) 143–147 |
| [3] | E. Ruijter, R.V.A. Orru. Multicomponent reactions-opportunities for the pharmaceutical industry. Drug Dis. Today:Technol. 10 (2013) e15–e20 |
| [4] | R.V.A. Orru, M. de Greef. Recent advances in solution-phase multicomponent methodology for the synthesis of heterocyclic compounds. Synthesis 2003 (2003) 1471–1499 |
| [5] | Drug Dis. Today:Technol.. The Hantzsch reaction I. Oxidative dealkylation of certain dihydropyridines. J. Org. Chem. 30 (1965) 1914–1916 |
| [6] | L.M. Wang, J. Sheng, L. Zhang, et al. Facile Yb(OTf)3 promoted one-pot synthesis of polyhydroquinoline derivatives through Hantzsch reaction. Tetrahedron 61 (2005) 1539–1543 |
| [7] | J.L. Donelson, R.A. Gibbs, S.K. De. An efficient one-pot synthesis of polyhydroquinoline derivatives through the Hantzsch four component condensation. J. Mol. Catal. A:Chem. 256 (2006) 309–311 |
| [8] | M. Maheswara, V. Siddaiah, G.L.V. Damu, C.V. Rao. An efficient one-pot synthesis of polyhydroquinoline derivatives via Hantzsch condensation using a heterogeneous catalyst under solvent-free conditions. ARKIVOC 2006 (2006) 201–206 |
| [9] | N.N. Karade, V.H. Budhewar, S.V. Shinde, W.N. Jadhav. L-Proline as an efficient organo-catalyst for the synthesis of polyhydroquinoline via multicomponent Hantzsch reaction. Lett. Org. Chem. 4 (2007) 16–19 |
| [10] | A. Kumar, R.A. Maurya. Bakers' yeast catalyzed synthesis of polyhydroquinoline derivatives via an unsymmetrical Hantzsch reaction. Tetrahedron Lett. 48 (2007) 3887–3890 |
| [11] | A. Kumar, R.A. Maurya. Synthesis of polyhydroquinoline derivatives through unsymmetric Hantzsch reaction using organocatalysts. Tetrahedron 63 (2007) 1946–1952 |
| [12] | S.R. Cherkupally, R. Mekala. p-TSA catalyzed facile and efficient synthesis of polyhydroquinoline derivatives through Hantzsch multi-component condensation. Chem. Pharm. Bull. 56 (2008) 1002–1004 |
| [13] | F.M. Moghaddam, H. Saeidian, Z. Mirjafary, A. Sadeghi. Rapid and efficient one-pot synthesis of 1,4-dihydropyridine and polyhydroquinoline derivatives through the Hantzsch four component condensation by zinc oxide. J. Iran. Chem. Soc. 6 (2009) 317–324 |
| [14] | S.B. Sapkal, K.F. Shelke, B.B. Shingate, M.S. Shingare. Nickel nanoparticle-catalyzed facile and efficient one-pot synthesis of polyhydroquinoline derivatives via Hantzsch condensation under solvent-free conditions. Tetrahedron Lett. 50 (2009) 1754–1756 |
| [15] | A. Khojastehnezhad, F. Moeinpour, A. Davoodnia. PPA-SiO2 catalyzed efficient synthesis of polyhydroquinoline derivatives through Hantzsch multicomponent condensation under solvent-free conditions. Chin. Chem. Lett. 22 (2011) 807–810 |
| [16] | N.K. Ladani, D.C. Mungra, M.P. Patel, R.G. Patel. Microwave assisted synthesis of novel Hantzsch 1,4-dihydropyridines, acridine-1,8-diones and polyhydroquinolines bearing the tetrazolo[1,5-a]quinoline moiety and their antimicrobial activity assess. Chin. Chem. Lett. 22 (2011) 1407–1410 |
| [17] | H. Khabazzadeh, E.T. Kermani, D. Afzali, A. Amiri, A. Jalaladini. Efficient one-pot synthesis of polyhydroquinoline derivatives using Cs2.5H0.5PW12O40 as a heterogeneous and reusable catalyst in molten salt media,. Arab. J. Chem. 5 (2012) 167–172 |
| [18] | R. Surasani, D. Kalita, A.V.D. Rao, K. Yarbagi, K.B. Chandrasekhar. FeF3 as a novel catalyst for the synthesis of polyhydroquinoline derivatives via unsymmetrical Hantzsch reaction. J. Fluor. Chem. 135 (2012) 91–96 |
| [19] | M. Tajbakhsh, E. Alaee, H. Alinezhad, et al. Titanium dioxide nanoparticles catalyzed synthesis of Hantzsch esters and polyhydroquinoline derivatives. Chin. J. Catal. 33 (2012) 1517–1522 |
| [20] | M. Shaquiquzzaman, S.A. Khan, M. Amir, M.M. Alam. Synthesis, anticonvulsant and neurotoxicity evaluation of some new pyrimidine-5-carbonitrile derivatives. Saudi Pharm. J. 20 (2012) 149–154 |
| [21] | M. Anzini, A. Cappelli, S. Vomero. Synthesis of 6-(4-methyl-1-piperazinyl)-7Hindeno[2,1-c]-quinoline derivatives as potential 5-HT receptor ligands. J. Heterocycl. Chem. 28 (1991) 1809–1812 |
| [22] | M. Yamato, Y. Takeuchi, K. Hashigaki, et al. Synthesis and antitumor activity of fused tetracyclic quinoline derivatives. J. Med. Chem. 32 (1989) 1295–1300 |
| [23] | L.W. Deady, J. Desneves, A.J. Kaye, et al. Synthesis and antitumor activity of some indeno[1,2-b]quinoline-based bis carboxamides. Bioorg. Med. Chem. 8 (2000) 977–984 |
| [24] | L.W. Deady, J. Desneves, A.J. Kaye, et al. Positioning of the carboxamide side chain in 11-oxo-11H-indeno[1,2-b]quinoline carboxamide anticancer agents:effects on cytotoxicity. Bioorg. Med. Chem. 9 (2001) 445–452 |
| [25] | A. Rampa, A. Bisi, F. Belluti, et al. Acetylcholinesterase inhibitors for potential use in Alzheimer's disease:molecular modeling, synthesis and kinetic evaluation of 11H-indeno-[1,2-b]-quinolin-10-ylamine derivatives. Bioorg. Med. Chem. 8 (2000) 497–506 |
| [26] | B. Venugopalan, C.P. Bapat, E.P. Desouza. Synthesis of 2- and 3-(4-chlorophenyl)-4-hydroxy-7-(4-trifluoromethylphenyl)-5,6,7,8-tetrahydroquinolin-5-one and 5,10-dihydro-11H-8-chloroindeno[1,2-b]quinolin-10,11-diones as antimalarials. Indian J. Chem. 31B (1992) 35–38 |
| [27] | A.A. Bekhit, O.A. El-Sayed, E. Aboulmagd, J.Y. Park. Tetrazolo[1,5-a]quinoline as a potential promising new scaffold for the synthesis of novel anti-inflammatory and antibacterial agents. Eur. J. Med. Chem. 39 (2004) 249–255 |
| [28] | L.W. Deady, J. Desneves, A.J. Kaye, et al. Ring-substituted 11-oxo-11Hindeno[1,2-b]quinoline-6-carboxamides with similar patterns of cytotoxicity to the dual topo Ⅰ/Ⅱ inhibitor DACA. Bioorg. Med. Chem. 7 (1999) 2801–2809 |
| [29] | A. Ryckebusch, D. Garcin, A. Lansiaux, et al. Synthesis, cytotoxicity, DNA interaction, and topoisomerase Ⅱ inhibition properties of novel indeno[2,1-c]quinolin-7-one and indeno[1,2-c]isoquinolin-5,11-dione derivatives. J. Med Chem. 51 (2008) 3617–3629 |
| [30] | C.H. Tseng, C.C. Tzeng, K.Y. Chung, et al. Synthesis and antiproliferative evaluation of 6-aryl-11-iminoindeno[1,2-c]quinoline derivatives. Bioorg. Med. Chem. 19 (2011) 7653–7663 |
| [31] | R.S. Upadhayaya, P.D. Shinde, A.Y. Sayyed, et al. Synthesis and structure of azolefused indeno[2,1-c]quinolines and their anti-mycobacterial properties. Org. Biomol. Chem. 8 (2010) 5661–5673 |
| [32] | A. Kumar, S. Sharma, V.D. Tripathi, et al. Design and synthesis of 2,4-disubstituted polyhydroquinolines as prospective antihyperglycemic and lipid modulating agents. Bioorg. Med. Chem. 18 (2010) 4138–4148 |
| [33] | R.J. Porter, R.J. Cereghino, G.D. Gladding, et al. Antiepileptic drug development program. Clevel. Clin. Q. 51 (1984) 293–295 |
| [34] | S.B. Wang, X.Q. Deng, Y. Zheng, et al. Synthesis and evaluation of anticonvulsant and antidepressant activities of 5-alkoxytetrazolo[1,5-c]thieno[2,3-e]pyrimidine derivatives. Eur. J. Med. Chem. 56 (2012) 139–144 |
| [35] | G. Pöch, S.N. Pancheva. Calculating slope and ED50 of additive dose-response curves, and application of these tabulated parameter values. J. Pharmacol. Toxicol. Methods 33 (1995) 137–145 |
| [36] | G. Saravanan, V. Alagarsamy, P. Dineshkumar. Anticonvulsant activity of novel 1-(morpholinomethyl)-3-substituted isatin derivatives. Bull. Fac. Pharm. Cairo Univ. 52 (2014) 115–124 |
| [37] | M.K. Ibrahim, K. El-Adl, A.A.Al-Karmalawy, Design, synthesis, molecular docking and anticonvulsant evaluation of novel 6-iodo-2-phenyl-3-substituted-quinazolin-4(3H)-ones, Bulletin of Faculty of Pharmacy, Cairo University. (2015). |
 2016, Vol. 27
2016, Vol. 27