Ionic liquids have attracted explosive research interest in the framework of green chemistry at recent time as environmental caring solvents due to their favorable properties like nonflammability,non-volatile,high thermal stability and easy to recycle [1]. Initially,ionic liquids are used as potential ‘‘green’’ alternative to volatile organic solvent because of their unique chemical and physical properties [2]. Recently,many attempts have been made to seek out functional ionic liquids through inclusion of additional functional groups as a part of the cation and/or anion i.e. task-specific ionic liquids [3]. The inclusion of functional groups can impart a particular ability to the ionic liquids i.e. solvent as well as catalyst or both [4]. Task-specific ionic liquids are extensively used as a catalyst for many type of reaction [5].
The efficient construction of highly biological active heterocyclic compounds without use of hazardous organic solvents and toxic catalyst is one of the most important tasks in the field of synthetic medicinal and green chemistry [6]. Multicomponent reactions (MCRs) have been proved to be a powerful and useful tool in modern synthetic chemistry as it provides rapid building of complex structure in a convergent and atom economical way [7]. Therefore developments of multicomponent reactions (MCRs) have attracted much attention from the advantage point of combinatorial chemistry [8]. In contrast,a multi step reaction involves a catalyst which may be highly toxic and may at times be difficult to be removed from the reaction mixture. A multi step reaction may require purification at each individual step [9].
The indole nucleus is a vastly resourceful heterocyclic scaffold present in various types of natural product and medicinal agent [10]. Isatin and its derivatives also have shown a variety of biological properties and are present in many natural products [11]. In addition it has been reported that biological activity could be enhanced to a greater extent by sharing indole 3-carbon atom in the formation of spiroindoline derivatives [12]. The spirooxindole systems (Fig. 1) are consequently found in many pharmacological agents and natural alkaloids with highly pronounced biological properties [13]. Moreover,spirooxindole derivatives is very important in synthetic medicinal chemistry due to showing a variety of pharmacological and biological activity such as anti-HIV [14],anti-tubercular [15],anti-cancer [16],anti-tumor [17] etc. therefore,for such synthesis and application of the compounds causing widespread concern.
|
Download:
|
| Fig. 1. Representatives of spirooxindole containing compounds. | |
Survey of literature data shows that several new pathways have been developed in the past few years for related of isatin, malononitrile and various types of carbonyl compound possessing a reactive α-methylene group in one pot multicomponent synthesis [18]. In this context,some catalysts have been recently reported to promote these condensations such as Mg(ClO4)2 [19] chitosan [20], meglumine [21],piperidine [22],NaBr [23],carbon-SO3H [24a].However,few methods have been develope dwithout catalyst such as water [25],propanol [26] glycerol [27] DMSO [28] at reflux temperature. Some of these methodologies are quite satisfactory, some of them require long reaction time to achieve reasonable yield.
On the other hand,there have been few reports about the synthesis of spirooxindole derivatives in ionic liquids such as bmim(OH)/chitosan/EtOH [29] (TBA acetate) [30] Ch- OSO3H]3W12PO40 [31],(H3N + CH2CH2OH) (HCOO-) [32],(SBDBU)Cl [33],EDDF-PEG60 [34],[BMIm]BF4 [35],DES [36]. Though these reported ionic liquids show good results,still the search for new task-specific ionic liquid is progressively in demand.
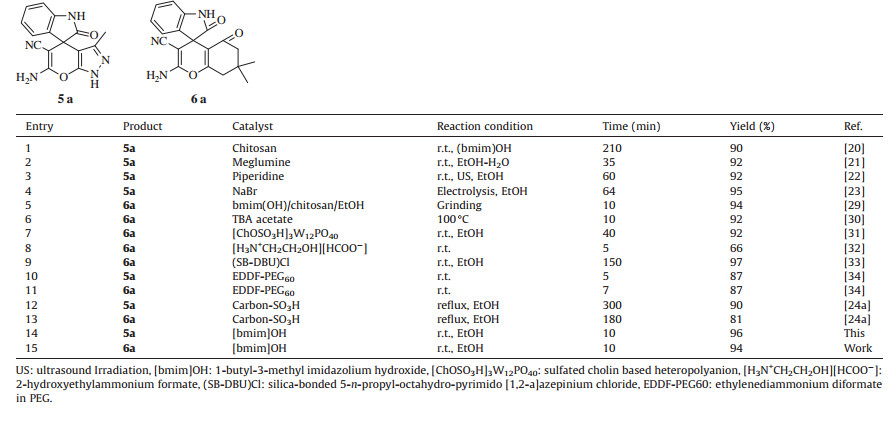
Finally,to prominent the efficiency and features of the present protocol in the synthesis of mono and newer bis spirooxindole derivatives; it was compared with some of the previously reported catalyst including some ionic liquids summarized results in Table 1. These reported protocols find certain merits of their own. All of them work on only mono-system,some of them required additional energy (i.e.,heating,ultrasonication or microwaves),some of them require long reaction time to achieve reasonable yield.
|
|
Table 1 Comparison with some reported methods. |
This comparison clearly highlights the present method is definitely advantageous to several of the others in terms of ionic liquid as reusable catalyst,reaction at room temperature,short reaction time,simple work up with high product yield and high purity,no solvent extraction used.
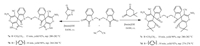
Considering the above importance and continuation of our work in organic synthetic methods [37],we report herein the synthesis of mono and newer bis spirooxindole derivatives in EtOH using [bmim]OH as a catalyst. The present protocol is a rapid and green methodology for the synthesis of mono spirooxindole derivatives using isatin,malononitrile and a-methylene carbonyl compounds (Scheme 1). In addition to that we are reporting a newer bis spirooxindoles derivative from 1,10-(butane-1,4-diyl)- bis(indoline-2,3-dione),1,10-(1,4-phenylenebis(methylene))bis(indoline-2,3-dione),malononitrile and a-methylene carbonyl compounds using task specific ionic liquid as a green,efficient and recyclable catalyst (Scheme 2).

|
Download:
|
| Scheme. 1. Synthesis of spirooxindoles. | |
|
Download:
|
| Scheme. 2. [bmim]OH catalyzed one-pot synthesis of various bis spirooxindoles (7a-d) at room temperature. | |
2. Experimental
All the chemicals were commercially available (Isatin,malononitrile,3-methyl-1H-pyrazol-5(4H)-one,dimedone purchase from Spectrochem India). Reactions were monitored by thin-layer chromatography (TLC) using silica gel-coated plates (Merck) and EtOAc/hexane solvent as the mobile phase. Spots were visualized under UV light. Melting points were recorded in open glass capillary method and are uncorrected. 1H NMR and 13C NMR were recorded on Bruker Avance-II spectrophotometer operating at 400 MHz and 100 MHz. The mass spectra were recorded under ESI mode with Waters micromass equipment (model Q-TOF MICROMASS). The ionic liquid [bmim]OH was synthesized according to the literature [38].
General experimental procedure for synthesis mono and bis spirooxindole derivatives (5a-f,6a-e and 7a-d) by a mixture of isatin (1 mmol),malononitrile (1 mmol) and the ionic liquid [bmim]OH (1 mmol) was stirred at room temperature. A solid product was observed. Then 2 mL of ethanol was added and stirred for 2 min after which carbonyl compound possessing a reactive amethylene group (1 mmol) was added. Immediately solid product was disappearedandthereactionmixturewas stirredfora periodof timeuntilthe completionofthe reaction (checked byTLC). The solid was observed during the reaction and filtered to get the crude productandwashedsuccessivelywith2 mLofethanoltoaffordpure product in good to excellent yield. The ionic liquid remained in the ethanol and reused up to five runs without further purification. Although this procedure was optimized with a 1 mmol,2 mmol and 5 mmol scale reactions and obtained same results.
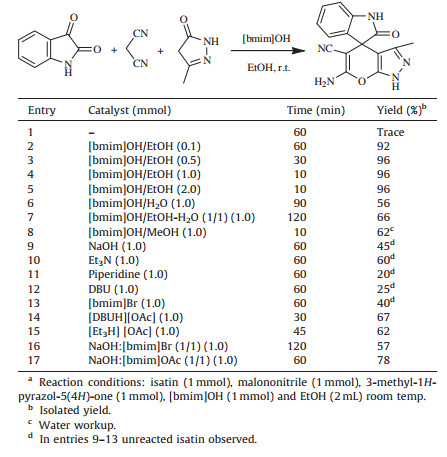
3. Results and discussionIn our initial study,we have carried out the reaction of isatin, malononitrile and 3-methyl-1H-pyrazol-5(4H)-one in ethanol to synthesized spirooxindoles in the absence of catalyst at room temperature. When the mixture was stirred at room temperature for 60 min,the product was obtained in very trace amount. Then, the reaction was investigated with different amounts catalyst (Table 2,entries 2-5). The best result was obtained,96% yield within 10 min,with 1 mmol of [bmim]OH (Table 2,entry 4). If the amount of catalyst was decreased from 1 mmol to 0.5 mmol and 0.1 mmol,the time of reaction is increase for conversion of 5a, while the use of 2 mmol of catalyst did not improve the yield and reaction time (Table 2,entry 5). The activity of the catalyst was tested with water (Table 2,entry 6) a solid was obtained which makes the separation of product very difficult. However,in EtOH- H2O (1:1) and MeOH,only the conversion of the starting materials is 66% (Table 2,entry 7) and 62% (Table 2,entry 8) into the product, respectively. It was also concluded that the use of EtOH as a reaction medium can greatly increase the rates of reactions with [bmim]OH due to its hydrophobic effects. We have tested some basic catalysts such as NaOH,Et3N,Piperidine,DBU; the moderate yield was obtained (Table 2,entries 9-12). We also compared [bmim]OH with the precursor [bmim]Br and the various ionic liquids such as [DBU[Ac] and [Et3NH][Ac] but we did not obtain satisfactory isolated yields. From optimization study,we conclude that hydroxide anion of the ionic liquid [bmim]OH plays a crucial role in the catalyst. We also compare with physical mixture of NaOH (0.1 mmol) with [bmim] Br (0.1 mmol) gave the trace amount of product after 24 h. If the amount of physical mixture was increases up to 1.00 mmol,the reaction time decreases and yield of spirooxindole increases up to 57%. However,when similar reactions with physical mixture of NaOH with [bmim]OAc were performed,the yield increased to 78% for 1.00 mmol. We constitute that result obtained by [bmim]OH promoted reaction better than others in terms of yields and reaction time. Therefore [bmim]OH is better than the above catalytic system. Hence,we decided to study the catalytic activity of the reaction system [bmim]OH:EtOH for the synthesis of 5a. After the separation of products,the catalyst containing ethanol was reused in the next run without further purification. The reaction medium can be reused up to five times without appreciable loss of activity with the excellent yields ranged from 96 to 84%,as shown in Fig. 2. The purity of recovered [bmim]OH further checked by FTIR,1H NMR,13C NMR and HPLC analysis (Figs. S1-S3 in Supporting information) and no changes found in the functional group of [bmim]OH.

|
Download:
|
| Fig. 2. Effect on product yield after each recycle and reuse of [bmim] OH/EtOH. | |
|
|
Table 2 Influence of different catalytic system for the synthesis of (5a) at room temperaturea. |
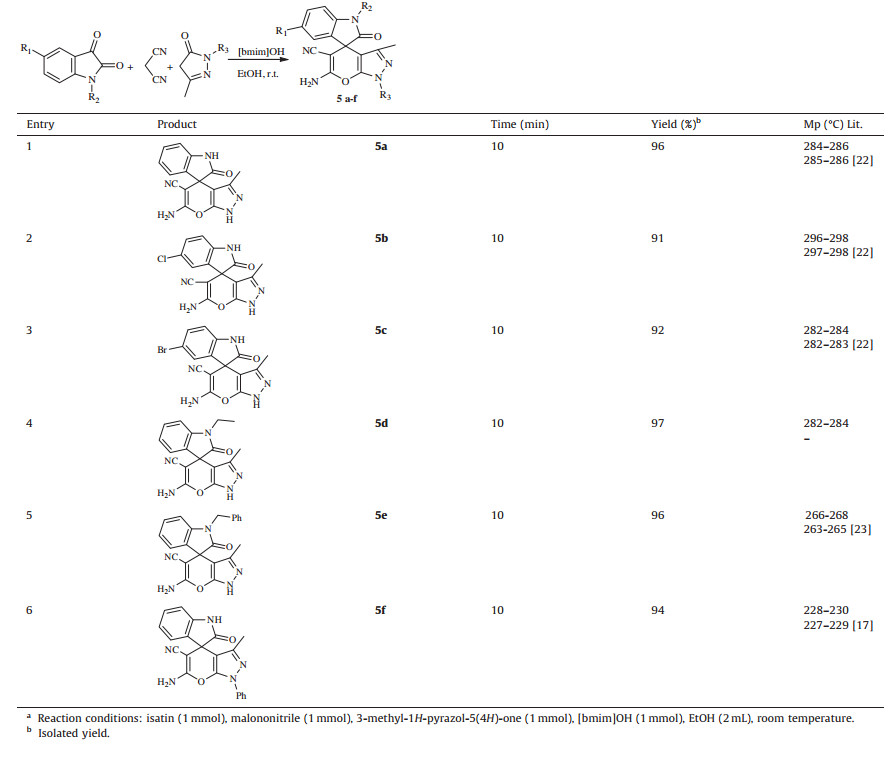
Thus,under this optimized reaction conditions,we used various substituted isatin derivatives with malononitrile and 3-methyl- 1H-pyrazol-5(4H)-one gave the desired product 5a-f with excellent yields (Table 3). 5-chloro,5-bromo and N-alkylated isatins were smoothly converted in to product within same reaction time. Encouraged by the results obtained with 3-methyl-1H-pyrazol- 5(4 H )-one,we extended our intension to investigate on dimedone gave the corresponding product 6a-e with excellent yields without formation of any side products (Table 4). In addition to these,we take efforts to synthesis newer bis spirooxindole derivatives from 1,10-(butane-1,4-diyl)bis(indoline-2,3-dione),1,10-(1,4-phenylenebis(methylene))bis(indoline-2,3-dione),malononitrile,3-methyl-1H-pyrazol-5(4H)-one and dimedone (Scheme 2). No significant substituent effect was observed on the reaction time and yield of product. Slightly higher time (15 min) was required to complete the formation of 6b-c and 7a. The future work will focused on the biological investigation of newer bis spirooxindole derivatives.
|
|
Table 3 [bmim]OH catalyzed one-pot synthesis of various spirooxindole (5a–f) at room temperaturea. |
|
|
Table 4 [bmim]OH catalyzed one-pot synthesis of various spirooxindole (6a–e) at room temperaturea. |
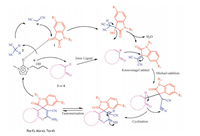
The probable reaction mechanism for [bmim]OH catalyzed synthesis of spirooxindole derivative is proposed in Scheme 3,in which Knoevenagel adduct is formed by adding malononitrile and isatin. On the other hand,hydroxide anion of the ionic liquid [bmim]OH abstract a proton from active methylene group,which undergoes Michael-addition with Knoevenagel adduct,followed by intramolecular cyclization and imine-amine tautomerization provides the target spiro compounds. Many of these products were reported compounds and further identified by comparison of their spectroscopic data and melting points with authentic samples and are in good agreement with the reported values. Some of the new interesting compounds were completely characterized by their spectroscopic techniques (1H NMR,13C NMR,Mass and HRMS)
|
Download:
|
| Scheme. 3. Plausible mechanism of the reaction. | |
4. Conclusion
In conclusion,we have developed highly efficient and practical method for the one-pot three-component reaction of isatin, malononitrile and carbonyl compounds with reactive α-methylene group for the synthesis of spirooxindole derivatives using [bmim]OH as an environmentally benign catalyst at room temperature. This protocol is not only limited for improvements in the reaction rates,yields and purity,but also avoiding the hazardous catalysts or solvents,elevated temperature,traditional chromatographic purifications and recrystallization.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2016.01.016.
| [1] |
(a) T. Welton, Room-temperature ionic liquids. solvents for synthesis and catalysis, Chem. Rev. 99(1999) 2071-2083; (b) P. Wasserscheid, W. Keim, Ionic liquids-new "solutions" for transition metal catalysis, Angew. Chem. Int. Ed. 39(2000) 3772-3789; (c) K. Gong, H. Wang, D. Fang, et al., Basic ionic liquid as catalyst for the rapid and green synthesis of substituted 2-amino-2-chromenes in aqueous media, Catal. Commun. 9(2008) 650-653; (d) H. Singh, S. Kumari, J.M. Khurana, A new green approach for the synthesis of 12-aryl-8, 9,10,12-tetrahydrobenzo[a]xanthenes-11-one derivatives using task specific acidic ionic liquid[NMP]H2PO4, Chin. Chem. Lett. 25(2014) 1336-1340. |
| [2] |
(a) R. Sheldon, Catalytic reactions in ionic liquids, Chem. Commun. (2001) 2399-2400;
(b) J.R. Harjani, S.J. Nara, M.M. Salunkhe, Lewis acidic ionic liquids for the synthesis of electrophilic alkenes via the knoevenagel condensation, Tetrahedron Lett. 43(2002) 1127-1130; (c) A.E. Visser, R.P. Swatloski, W.M. Reichert, et al., Task-specific ionic liquids for the extraction of metal ions from aqueous solutions, Chem. Commun. (2001) 135-136. |
| [3] | S.G. Lee. Functionalized imidazolium salts for task-specific ionic liquids and their applications. Chem. Commun. (2006) 1049–1063 |
| [4] |
(a) S. Chowdhury, R.S. Mohan, J.L. Scott, Reactivity of ionic liquids, Tetrahedron 63(2007) 2363-2389;
(b) W. Bao, Z. Wang, An effective synthesis of bromoesters from aromatic aldehydes using tribromide ionic liquid based on L-prolinol as reagent and reaction medium under mild conditions, Green Chem. 8(2006) 1028-1033; (c) D. Zhao, M. Wu, Y. Kou, et al., Ionic liquids:applications in catalysis, Catal. Today 74(2002) 157-189; (d) J. Dupont, R.F. de Souza, P.A.Z. Suarez, Ionic liquid (molten salt) phase organometallic catalysis, Chem. Rev. 102(2002) 3667-3692; (e) K. Qiao, C. Yakoyama, Novel acidic ionic liquids catalytic systems for friedel crafts alkylation of aromatic compounds with alkenes, Chem. Lett. 33(2004) 472-473; (f) W. Sun, C.G. Xia, H.W. Wang, Synthesis of aziridines from imines and ethyl diazoacetate in room temperature ionic liquids, Tetrahedron Lett. 44(2003) 2409-2411. |
| [5] |
(a) J.H. Davis, Task-specific ionic liquids, Chem. Lett. 33(2004) 1072-1077;
(b) T. Akiyama, A. Suzuki, K. Fuchibe, Mannich-type reaction promoted by an ionic liquid, Synlett 33(2005) 1024-1026; (c) B.C. Ranu, S. Banerjee, A. Das, Catalysis by ionic liquids:cyclopropyl carbonyl rearrangements catalyzed by[pmim]Br under organic solvent free conditions, Tetrahedron Lett. 47(2006) 881-884; (d) J.P. Hallett, T. Welton, Room-temperature ionic liquids:solvents for synthesis and catalysis 2, Chem. Rev. 111(2011) 3508-3576. |
| [6] |
(a) Y. Gu, Multicomponent reactions in unconventional solvents:state of the art, Green Chem. 14(2012) 2091-2128;
(b) L. Hu, O. Ramstrom, Silver-catalyzed dynamic systemic resolution of α-iminonitriles in a 1,3-dipolar cycloaddition process, Chem. Commun. 50(2014) 3792-3794. |
| [7] |
(a) X. Li, Y. Zhao, H. Qu, et al., Organocatalytic asymmetric multicomponent reactions of aromatic aldehydes and anilines with β-ketoesters:facile and atomeconomical access to chiral tetrahydropyridines, Chem. Commun. 49(2013) 1401-1403;
(b) E. Ruijter, R. Scheffelaar, R. Orru, Multicomponent reaction design in the quest for molecular complexity and diversity, Angew. Chem. Int. Ed. 50(2011) 6234-6246; (c) A. Domling, Recent developments in isocyanide based multicomponent reactions in applied chemistry, Chem. Rev. 106(2006) 17-89. |
| [8] |
(a) R.V.A. Orru, M. de Greef, Recent advances in solution-phase multicomponent methodology for the synthesis of heterocyclic compounds, Synthesis 10(2003) 1471-1499;
(b) G. Balme, E. Bossharth, N. Monteiro, Pd-assisted multicomponent synthesis of heterocycles, Eur. J. Org. Chem. 21(2003) 4101-4111; (c) S. Brase, C. Gil, K. Knepper, The recent impact of solid-phase synthesis on medicinally relevant benzoannelated nitrogen heterocycles, Bioorg. Med. Chem. 10(2002) 2415-2437; (d) A. Domling, I. Ugi, Multicomponent reactions with isocyanides, Angew. Chem. Int. Ed. 39(2000) 3168-3170. |
| [9] |
(a) M. Srivastava, P. Rai, J. Singh, et al., An environmentally friendlier approachionic liquid catalysed, water promoted and grinding induced synthesis of highly functionalised pyrazole derivatives, RSC Adv. 3(2013) 16994-16998;
(b) L.R. Wen, Z.R. Li, M. Li, et al., Solvent-free and efficient synthesis of imidazo[1,2-a]pyridine derivatives via a one-pot three-component reaction, Green Chem. 14(2012) 707-716; (c) H. Chen, D. Shi, Efficient one-pot synthesis of spiro[indoline-3,4'-pyrazolo[3,4-e] [1,4] thiazepine]dione via three-component reaction, Tetrahedron 67(2011) 5686-5692. |
| [10] |
(a) G. Bartoli, G. Bencivenni, R. Dalpozzo, Organocatalytic strategies for the asymmetric functionalization of indoles, Chem. Soc. Rev. 39(2010) 4449-4465;
(b) L. Joucla, L. Djakovitch, Transition metal-catalysed, direct and site-selective N1-, C2- or C3-arylation of the indole nucleus:20 years of improvements, Adv. Synth. Catal. 351(2009) 673-714; (c) M. Bandini, A. Eichholzer, Catalytic functionalization of indoles in a new dimension, Angew Chem. Int. Ed. 48(2009) 9608-9644; (d) S. Cacchi, G. Fabrizi, Synthesis and functionalization of indoles through palladium-catalyzed reactions, Chem. Rev. 105(2005) 2873-2920; (e) Y. Kamano, H.P. Zhang, Y. Ichihara, et al., Convolutamydine a, a novel bioactive hydroxyoxindole alkaloid from marine bryozoan amathia convolute, Tetrahedron Lett. 36(1995) 2783-2784. |
| [11] |
(a) J. Xue, Y. Zhang, X.I. Wang, et al., Photoinduced reactions of 1-acetylisatin with phenylacetylenes, Org. Lett. 2(2000) 2583-2586;
(b) D.A. Klumpp, K.Y. Yeung, G.K.S. Prakash, et al., preparation of 3,3-diaryloxindoles by superacid-induced condensations of isatins and aromatics with a combinatorial approach, J. Org. Chem. 63(1998) 4481-4484. |
| [12] |
(a) J.F.M. Da Silva, S.J. Garden, A.C. Pinto, The chemistry of isatins:a review from 1975 to 1999, J. Braz. Chem. Soc. 12(2001) 273-324;
(b) A.H. Abdel, E.M. Keshk, M.A. Hannaand, et al., Synthesis and evaluation of some new spiro indoline-based heterocycles as potentially active antimicrobial agents, Bioorg. Med. Chem. 12(2004) 2483-2488. |
| [13] |
(a) F. Zhou, Y.L. Liu, J. Zhou, Catalytic asymmetric synthesis of oxindoles bearing a tetrasubstituted stereocenter at the C-3 position, Adv. Synth. Catal. 352(2010) 1381-1407;
(b) C.V. Galliford, K.A. Scheidt, Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents, Angew. Chem. Int. Ed. 46(2007) 8748-8758; (c) H. Lin, S.J. Danishefsky, Gelsemine:a thought-provoking target for total synthesis, Angew. Chem. Int. Ed. 42(2003) 36-51; (d) J. Ma, S.M. Hecht, Javaniside, a novel DNA cleavage agent from Alangium javanicum having an unusual oxindole skeleton, Chem. Commun. (2004) 1190-1191; (e) T.H. Kang, K. Matsumoto, M. Tohda, et al., Pteropodine and isopteropodine positively modulate the function of rat muscarinic M1 and 5-HT2 receptors expressed in Xenopus oocyte, Eur. J. Pharmacol. 444(2002) 39-45; (f) P.R. Sebahar, R.M. Williams, The asymmetric total synthesis of (+)- and (-)-spirotryprostatin B, J. Am. Chem. Soc. 122(2000) 5666-5667. |
| [14] | G. Kumari, Nutan, M. Modi, et al. Rhodium(Ⅱ) acetate-catalyzed stereoselective synthesis, SAR and anti-HIV activity of novel oxindoles bearing cyclopropane ring. Eur. J. Med. Chem. 46 (2011) 1181–1188 |
| [15] | V.V. Vintonyak, K. Warburg, H. Kruse, et al. Identification of thiazolidinones spirofused to indolin-2-ones as potent and selective inhibitors of the mycobacterium tuberculosis protein tyrosine phosphatase B. Angew. Chem. Int. Ed. Engl. 49 (2010) 5902–5905 |
| [16] | B. Yu, D.Q. Yu, H.M. Liu. Spirooxindoles:promising scaffolds for anticancer agents. Eur. J. Med. Chem. 97 (2015) 673–698 |
| [17] | Y. Tian, S. Nam, L. Liu, et al. Spirooxindole derivative SOID-8 induces apoptosis associated with inhibition of JAK2/STAT3 signaling in melanoma cells. PLoS ONE (2012) e49306 |
| [18] |
(a) Y. Li, H. Chen, C. Shi, et al., Efficient one-pot synthesis of spirooxindole derivatives catalyzed by L-proline in aqueous medium, J. Comb. Chem. 12(2010) 231-237;
(b) L.M. Wang, N. Jiao, J. Qiu, et al., Sodium stearate-catalyzed multicomponent reactions for efficient synthesis of spirooxindoles in aqueous micellar media, Tetrahedron 66(2010) 339-343; (c) M. Dabiri, M. Bahramnejad, M. Baghbanzadeh, Ammonium salt catalyzed multicomponent transformation:simple route to functionalized spirochromenes and spiroacridines, Tetrahedron 65(2009) 9443-9447; (d) S. Gao, C.H. Tsai, C. Tseng, et al., Fluoride ion catalyzed multicomponent reactions for efficient synthesis of 4H-chromene and N-arylquinoline derivatives in aqueous media, Tetrahedron 64(2008) 9143-9149; (e) Y.M. Litvinov, V.Y. Mortikov, A.M. Shestopalov, Versatile three-component procedure for combinatorial synthesis of 2-aminospiro[(3'H)-indol3',4-(4H)-pyrans], J. Comb. Chem. 10(2008) 741-745; (f) G. Shanthi, G. Subbulakshmi, P.T. Perumal, A new InCl3-catalyzed facile and efficient method for the synthesis of spirooxindoles under conventional and solvent-free microwave conditions, Tetrahedron 63(2007) 2057-2063; (g) R.G. Redkin, L.A. Shemchuk, V.P. Chernykh, et al., Synthesis and molecular structure of spirocyclic 2-oxindole derivatives of 2-amino-4H-pyran condensed with the pyrazolic nucleus, Tetrahedron 63(2007) 11444-11450; (h) Y. Caibo, G. Xuepin, W. Shenghua, et al., Green catalyzed synthesis method of spirooxindole derivative by using basic ionic liquid catalyst, CN 103833764 A 20140604. |
| [19] | C. Wu, R. Shen, J. Chen, et al. An efficient method for multicomponent synthesis of spiro. Bull. Korean Chem. Soc. 34 (2013) 2431–2435 |
| [20] | P. Rai, M. Srivastava, J. Singh, et al. Chitosan/ionic liquid forms a renewable and reusable catalyst system used for the synthesis of highly functionalized spiro derivatives. New J. Chem. 38 (2014) 3181–3186 |
| [21] | R.Y. Guo, Z.M. An, L.P. Mo, et al. Meglumine promoted one-pot, four-component synthesis of pyranopyrazole derivatives. Tetrahedron 69 (2013) 9931–9938 |
| [22] | Y. Zou, Y. Hu, H. Liu, et al. Rapid and efficient ultrasound-assisted method for the combinatorial synthesis of spiro. ACS Comb. Sci. 14 (2012) 38–43 |
| [23] | M.N. Elinson, A.S. Dorofeev, F.M. Miloserdov, et al. Electrocatalytic multicomponent assembling of isatins, 3-methyl-2-pyrazolin-5-ones and malononitrile:facile and convenient way to functionalized spirocyclic. Mol. Divers. 13 (2009) 47–52 |
| [24] |
(a) B.M. Rao, G.N. Reddy, T.V. Reddy, et al., Carbon-SO3H:a novel and recyclable solid acid catalyst for the synthesis of spiro[4H-pyran-3,30-oxindoles], Tetrahedron Lett. 54(2013) 2466-2471;
(b) L.A. Shemchuk, V.P. Chernykh, R.G. Redkin, Synthesis of fused 2'-amino-3'-Rspiro-[indole-3,4'-pyran]-2(1H)-ones, Russ. J. Org. Chem. 44(2008) 1789-1794. |
| [25] | L. Zhao, B. Zhou, Y. Li. An efficient one-pot three-component reaction for synthesis of spirooxindole derivatives in water media under catalyst-free condition. Heteroat. Chem. 22 (2011) 673–677 |
| [26] | M.N. Elinson, A.I. Ilovaisky, V.M. Merkulova, et al. Non-catalytic thermal multicomponent assembling of isatin, cyclic CH-acids and malononitrile:an efficient approach to spirooxindole scaffold. Mendeleev Commun. 22 (2012) 143–144 |
| [27] | H.R. Safaei, M. Shekouhy, S. Rahmanpur, et al. Glycerol as a biodegradable and reusable promoting medium for the catalyst-free one-pot three component synthesis of 4H-pyrans. Green Chem. 14 (2012) 1696–1704 |
| [28] | T. Ponpandian, S. Muthusubramanian. One-pot, catalyst-free synthesis of spirooxindole and 4H-pyran derivatives. Synth. Commun. 44 (2014) 868–874 |
| [29] | M. Srivastava, P. Rai, J. Singh, et al. Bmim(OH)/chitosan/C2H5OH synergy:grinding induced, a new route for the synthesis of spirooxindole and its derivatives. RSC Adv. 4 (2014) 30592–30597 |
| [30] | S. Riyaz, A. Indrasena, A. Naidu, et al. Novel and thermally stable ionic liquid (TBA acetate) for domino reaction:synthesis of spirooxindoles via new mechanistic way,. Indian J. Chem. (2014) 1442–1447 |
| [31] | S.P. Satasia, P.N. Kalaria, J.R. Avalani, et al. An efficient approach for the synthesis of spirooxindole derivatives catalyzed by novel sulfated choline based heteropolyanion at room temperature. Tetrahedron 70 (2014) 5763–5767 |
| [32] | H.R. Shaterian, M. Arman, F. Rigi. Domino knoevenagel condensation, Michael addition, and cyclization using ionic liquid, 2-hydroxyethylammonium formate, as a recoverable catalyst. J. Mol. Liq. 158 (2011) 145–150 |
| [33] | A. Hasaninejada, N. Golzara, M. Beyrati, et al. Silica-bonded 5-n-propyl-octahydro-pyrimido[1, 2-a] azepinium chloride (SB-DBU)Cl as a highly efficient, heterogeneous and recyclable silica-supported ionic liquid catalyst for the synthesis of benzo[b]pyran, bis(benzo[b]pyran) and spiro-pyran derivatives. J. Mol. Catal., A Chem. 372 (2013) 137–150 |
| [34] | A. Thakur, M. Tripathi, U.C. Rajesh. Ethylenediammonium diformate (EDDF) in PEG600:an efficient ambiphilic novel catalytic system for the one-pot synthesis of 4H-pyrans via knoevenagel condensation. RSC. Adv. 3 (2013) 18142–18148 |
| [35] | K. Rad-Moghadam, L. Youseftabar-Miri. Ambient synthesis of spiro[4H-pyranoxindole] derivatives under[BMIm]BF4 catalysis. Tetrahedron 67 (2011) 5693–5699 |
| [36] | N. Azizi, S. Dezfooli, M.M. Hashemi. Greener synthesis of spirooxindole in deep eutectic solvent. J. Mol. Liq. 194 (2014) 62–67 |
| [37] |
(a) Y.B. Wagh, Y.A. Tayade, S.A. Padvi, et al., A cesium fluoride promoted efficient and rapid multicomponent synthesis of functionalized 2 amino-3-cyano-4Hpyran and spirooxindole derivatives, Chin. Chem. Lett. 26(2015) (2015) 1273-1277;
(b) Y.A. Tayade, S.A. Padvi, Y.B. Wagh, et al., β-Cyclodextrin as a supramolecular catalyst for the synthesis of dihydropyrano[2,3-c]pyrazole and spiro[indoline-3,4'-pyrano[2,3-c]pyrazole] in aqueous medium, Tetrahedron Lett. 56(2015) 2441-2447; (c) Y.A. Tayade, D.R. Patil, Y.B. Wagh, et al., An efficient synthesis of 3-indolyl-3 hydroxy oxindoles and 3,3-di(indolyl)indolin-2-ones catalyzed by sulfonated β-CD as a supramolecular catalyst in water, Tetrahedron Lett. 56(2015) 666-673; (d) A.D. Jangale, P.K. Kumavat, Y.B. Wagh, et al., Green process development for the synthesis of aliphatic symmetrical N,N'-disubstituted thiourea derivatives in aqueous medium, Synth. Commun. 45(2015) 236-244; (e) D.R. Patil, Y.B. Wagh, P.G. Ingole, et al., β-Cyclodextrin-mediated highly efficient[2+3] cycloaddition reactions for the synthesis of 5-substituted 1-H tetrazoles, New J. Chem. 37(2013) 3261-3266; (f) D.R. Patil, D.S. Dalal, Biomimetic approach for the synthesis of N,N' diarylsubstituted formamidines catalyzed by β-cyclodextrin in water, Chin. Chem. Lett. 23(2012) 1125-1128. |
| [38] | B.C. Ranu, S. Banerjee. Ionic liquid as catalyst and reaction medium. the dramatic influence of a task-specific ionic liquid, [bmim]OH, in michael addition of active methylene compounds to conjugated ketones, carboxylic esters, and nitriles. Org. Lett. 7 (2005) 3049–3052 |
 2016, Vol. 27
2016, Vol. 27