b Department of Pharmacy, IK Gujral Punjab Technical University, Jalandhar 144001, Punjab, India ;
c Department of Industrial Chemistry, Guru Nanak Khalsa College, Yamuna Nagar 135001, Haryana, India ;
d IEC School of Pharmacy, IEC University, Baddi 173205, Himachal Pardesh, India
At present,diverse classes of compounds posses anti-inflammatory activity including non-steroidal anti-inflammatory drugs (NSAIDS),steroidal anti-inflammatory agents or synthetic forms of natural cortisol (glucocorticoids),pharmaceutical biologics and many more. Although the drug treatment has been improved to steadily but yet,it is still a challenge for the pharmaceutical chemists to identify more effective,potent,less toxic therapeutic agents to treat as well as reduce the signs and symptoms of acute inflammation and chronic inflammatory diseases. In addition,it is well known that bacterial infections often produce pain and inflammation. In normal practice,two groups of agents (chemotherapeutic and anti-inflammatory) are prescribed simultaneously to treat bacterial infections with inflammatory disorders. Unfortunately,none of the drugs possesses these three activities in a single component. Therefore,our aim is to find a compound having dual antimicrobial and anti-inflammatory activities. Here, we are presenting some 5- substituted imidazolone analogs with comparable antibacterial and anti-inflammatory potencies.
5-Imidazolone is a five-membered heterocyclic ring system having three carbon and two nitrogen atoms at the 1 and 3 positions with a carbonyl group at the 5 position. Several imidazolone analogs were also found to be associated with diverse biological activities including analgesic and anti-inflammatory [1, 2, 3, 4],CNS depressant [5],monoamine oxidase (MAO) inhibitory [6],anticonvulsant [6, 7],immunomodulator [8],antianthelmintic [9],anticancer [10],cardiovascular [11, 12],antimicrobial [13, 14] etc. Previously the imidazolones have been prepared by heating a mixture of 5-oxazolones derivatives with aromatic and substituted aromatic amine in the presence of pyridine for 10-15 h. The yield of imidazolones was very poor and the reaction required a long time [15, 16, 17, 18, 19]. In this report we have introduced some new imidazolone analogs,synthesized by the condensation of 5-oxazolones analogs with substituted aromatic amines in the presence of anhydrous pyridine under microwave irradiation. The structures of all the prepared analogs were determined using IR,1H NMR and all the prepared analogs were screened for antimicrobial and anti-inflammatory potential.
2. Experimental 2.1. ChemistryAll melting points were determined by the open capillary tube method and are uncorrected. IR spectra were recorded on a Perkin Elmer RX1 spectrophotometer using KBr pellets and are expressed in cm-1. The 1H NMR and 13C NMR spectra were recorded on a Brucker 300 MHz spectrometer in (CDCl3) using TMS as an internal reference and chemical shifts were measured in δ ppm. The progress of the reaction was monitored by TLCanalysis using 0.2 mm thickness aluminum sheets pre-coated with silica gel Merck 60F 254 and visualization was done using iodine/UV lamp. The solvents were removed under reduced pressure using a Buchi rotary evaporator.
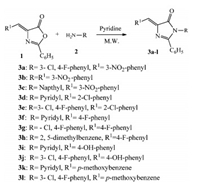
2.2. General procedure for the synthesis of title compounds 3a-lA series of 5-substituted imidazolones were synthesized by the condensation of different 5-substituted oxazolones (1 mmol) and substituted aromatic amines (1.1 mmol) in anhydrous pyridine under solvent free conditions in a microwave reactor (Scheme 1). The reaction was completed in 10-15 min. The completion of reaction was monitored by TLC analysis and then 5 mL of icecooled 5% HCl in water was added and the mixture was left for overnight. The resultant solids were collected and washed with water. The resultant solid was crystallized from ethanol,filtered and dried to afford titled compounds 3a-l.
|
Download:
|
| Scheme. 1. Preparation of 5-substitituted imidazolones. | |
4-(3-Nitrobenzylidene)-1-(3-chloro-4-fluorophenyl)-2-phenyl-1H-imidazol-5(4H)-one 3a: Yield: 70%. Mp 214-216 ℃; IR (KBr,cm-1): 1615 (C=N),1597 (C=C),1645 (C=O). 1H NMR (CDCl3, 100 MHz): δ 7.25 (s,1H),6.90-8.25 (m,12H). 13C NMR (CDCl3, 300 MHz): δ 170,158.4,148,136.2,132.4,130.2,128.5,121,117, 108.
4-(3-Nitrobenzylidene)-1-(3-nitrophenyl)-2-phenyl-1H-imidazol-5(4H)-one 3b: Yield: 72%. Mp 217-219 ℃; IR (KBr,cm-1): 1620 (C=N),1598 (C=C),1655 (C=O). 1H NMR (CDCl3,300 MHz): δ 7.25 (s,1H),7.31-8.54 (m,13H). 13C NMR (CDCl3,100 MHz): δ 170, 164.4,147,136,133.2,132.5,130,129.5,128,126,121,116,106.
4-(3-Nitrobenzylidene)-1-(naphthalen-1-yl)-2-phenyl-1H-imidazol-5(4H)-one 3c: Yield: 67%. Mp 223-224 ℃; IR (KBr,cm-1):1605 (C=N),1590 (C=C),1660 (C=O). 1H NMR (CDCl3,300 MHz): δ 7.20 (s,1H),6.80-8.25 (m,16H). 13C NMR (CDCl3,100 MHz): δ 170.2,164.1,148.2,141.2,136.5,134.2,132.5,130.0,128.4,126.2, 124.4,121.2,119.0,109.3,108.4.
4-(2-Chlorobenzylidene)-2-phenyl-1-(pyridin-2-yl)-1H-imidazol-5(4H)-one 3d: Yield: 77%. Mp 216-217 ℃; IR (KBr,cm-1): 1610 (C=N),1594 (C=C),1655 (C=O). 1H NMR (CDCl3,300 MHz): δ 7.7 (s,1H),6.80-8.10 (m,13H). 13C NMR (CDCl3,100 MHz): δ 170.1, 164.3,147.5,138.2,133.1,131.2,130.4,128.3,126.2,113.4,109.5, 108.3.
4-(2-Chlorobenzylidene)-1-(3-chloro-4-fluorophenyl)-2-phenyl-1H-imidazol-5(4H)-one 3e: Yield: 79%. Mp 210-212 ℃; IR (KBr,cm-1): 1600 (C=N),1580 (C=C),1650 (C=O). 1H NMR (CDCl3, 300 MHz): δ 7.8 (s,1H),6.80-7.70 (m,12H). 13C NMR (CDCl3, 100 MHz): δ 170.3,164.0,158.3,133.2,131.2,129.4,128.5,128.2, 126.5,126.2,123.3,121.0,117.1,108.2.
4-(4-Fluorobenzylidene)-2-phenyl-1-(pyridin-2-yl)-1H-imidazol-5(4H)-one 3f: Yield: 76%. Mp 238-240 ℃; IR (KBr,cm-1): 1610 (C=N),1596 (C=C),1645 (C=O). 1H NMR (CDCl3,300 MHz): δ 7.6 (s,1H),6.70-8.10 (m,13H). 13C NMR (CDCl3,100 MHz): δ 170.2, 164.3,162.2,148.1,138.2,130.6,130.2,128.4,128.0,126.1,115.4, 113.1,109.2,108.1.
4-(4-Fluorobenzylidene)-1-(3-chloro-4-fluorophenyl)-2-phenyl-1H-imidazol-5(4H)-one 3g: Yield: 68%. Mp 228-230 ℃; IR (KBr,cm-1): 1620 (C=N),1595 (C=C),1650 (C=O). 1H NMR (CDCl3, 300 MHz): δ 7.7 (s,1H),6.90-7.60 (m,12H). 13C NMR (CDCl3, 100 MHz): δ 170.2,164.3,162.3,158.4,130.4,129.6,128.5,128.2, 126.1,123.4,121.0,117.1,115.4,108.3.
4-(4-Fluorobenzylidene)-1-(2,6-dimethylphenyl)-2-phenyl- 1H-imidazol-5(4H)-one 3h: Yield: 60%. Mp 238-239 ℃; IR (KBr, cm-1): 1618 (C=N),1590 (C=C),1645 (C=O). 1H NMR (CDCl3, 300 MHz): δ 2.3 (s,6H),7.6 (s,1H),6.80-7.60 (m,12H). 13C NMR (CDCl3,100 MHz): δ 170.1,164.2,162.3,142.3,134.2,130.4,128.6, 128.0,126.3,124.4,115.2,108.1.
4-(4-Hydroxybenzylidene)-2-phenyl-1-(pyridin-2-yl)-1H-imidazol-5(4H)-one 3i: Yield: 62%. Mp 234-235 ℃; IR (KBr,cm-1): 1620 (C=N),1590 (C=C),1650 (C=O). 1H NMR (CDCl3,300 MHz): δ 5.0 (s,1H),7.6 (s,1H),6.70-8.10 (m,13H). 13C NMR (CDCl3, 100 MHz): δ 170.2,164.2,157.3,147.4,138.3,130.2,128.6,127.0, 126.3,115.2,113.1,109.2,108.4.
4-(4-Hydroxybenzylidene)-1-(3-chloro-4-fluorophenyl)-2- phenyl-1H-imidazol-5(4H)-one 3j: Yield: 64%. Mp 242-243 ℃; IR (KBr,cm-1): 1610 (C=N),1592 (C=C),1655 (C=O). 1H NMR (CDCl3, 300 MHz): δ 5.0 (s,1H),7.6 (s,1H),6.70-7.80 (m,12H). 13C NMR (CDCl3,100 MHz): δ 170.1,164.2,157.2,130.2,128.6,127.3,126.1, 123.4,121.3,117.4,115.4,108.1.
4-(4-Methoxybenzylidene)-2-phenyl-1-(pyridin-2-yl)-1H-imidazol-5(4H)-one 3k: Yield: 70%. Mp 235-237 ℃; IR (KBr): 3105 (Ar C-H),1620 (C=N),1594 (C=C),1645 (C=O) cm-1. 1H NMR (CDCl3, 300 MHz): δ 3.70 (s,3H),7.5 (s,1H),6.70-8.10 (m,13H). 13C NMR (CDCl3,100 MHz): δ 170.2,164.1,159.2,147.5,138.3,130.0,128.3, 127.4,126.1,114.2,113.4,109.4,108.1,55.1.
4-(4-Methoxybenzylidene)-1-(3-chloro-4-fluorophenyl)-2- phenyl-1H-imidazol-5(4H)-one 3l: Yield: 72%. Mp 225-227 ℃; IR (KBr,cm-1): 3100 (Ar C-H),1615 (C=N),1590 (C=C),1660 (C=O). 1H NMR (CDCl3,300 MHz): δ 3.70 (s,3H),7.6 (s,1H),6.70-7.70 (m, 12H). 13C NMR (CDCl3,300 MHz): δ 170.1,164.1,159.4,158.4, 130.2,128.4,127.2,126.1,123.4,121.0,117.2,114.4,108.1,55.2.
2.3. Biological activity 2.3.1. Antimicrobial activityThe in vitro antimicrobial potential of all the prepared analogs was carried out at Institute of Microbial Technology (CSIR), Chandigarh-160036 (India) using the agar plate diffusion antimicrobial bioassay. The compounds were tested at 5000 mg/mL concentration (DMSO) and the activity was determined by measuring the zone of inhibition. The positive control strength is specified i.e. 50 mg/mL of the stock solution was used for bioassay. The antibacterial and antifungal potential of the prepared analogs 3a-l were compared with standard drugs ampicillin (200 mg/mL) and Amphotericin B (500 mg/mL),respectively. Activity was determined by measuring the diameter of zones showing complete inhibition (mm). Growth inhibition was calculated with reference to positive control.
2.3.2. Anti-inflammatory activityAdult male Wistar rats (150-180 g) from the animal house of Guru Gobind Singh College of Pharmacy,Yamuna Nagar (Regn. No. 873/PO/ac/05/CPCSEA) were used throughout the work. They were kept under standard conditions of light and temperature with free access to food and water. The animals were randomly divided into groups of six rats each. The paw edema was induced by sub-plantar injection of 50 mL of 1% carrageenan solution in saline (0.9%). Indomethacin and the compounds 3a-l were dissolved in DMSO and injected subcutaneously in different dose levels of 1 and 10 mg/kg body weight respectively,1 h prior to the carrageenan injection. DMSO was injected to the control group. The volume of paw edema (in mL) was determined by means of a water plethysmometer immediately after the injection of carrageenan and 4 h later. The percentage protection against inflammation was calculated as follows: (Vc - Vd)/Vc × 100,where Vc is the increase in paw volume in the absence of the test compound (control) and Vd is the increase of paw volume after injection of the test compound. Data were expressed as means ± SEM. Significant differences between the control and the treated groups were obtained using the Student’s t-test. The differences were considered significant when p < 0.001.
2.3.3. Ulcerogenic activityMale albino rats (120-150 g) were fasted for 12 h prior to the administration of the compounds. The animals were divided into five equal groups,of four animals. The control group received 0.2 mL DMSO orally,reference groups received 5 mg/kg indomethacin and test groups received 10 mg/kg tested compounds orally for three successive days. Animals were sacrificed by diethyl ether 6 h after the last dose and the stomach was removed. An opening at the greater curvature was made and the stomach was cleaned by washing with cold saline and examined for ulceration. The number and diameter of discrete areas of damage in the glandular mucosa were scored. The ulcer score was calculated as: 0.0—normal (no injury); 0.5—latent injury; 1.0—slight injury (two to three dotted lines); 2.0—severe injury (continuous lined injury or five to six dotted injuries); 3.0—very severe injury (several continuous lined injuries); 4.0—widespread lined injury.
3. Results and discussion 3.1. ChemistryOne series of novel 5-substituted imidazolone have been synthesized in an attempt to find new compounds having both antimicrobial and anti-inflammatory activities. The synthetic pathway leading to the titled compounds is given in Scheme 1. The targeted 5-substituted imidazolone analogs 3a-l were prepared by the reaction of the substituted oxazolones 1 with various substituted aromatic amines 2 in the presence of anhydrous pyridine under microwave irradiation. The purity and structures of all the synthesized compounds have been elucidated on the basis of their spectral data including IR and 1H NMR.
3.2. Biological evaluation 3.2.1. Antibacterial activityAll the synthesized compounds 3a-l were tested against three Gram-positive bacterial strains i.e. Micrococcus luteus MTCC 2470, Staphylococcus aureus MTCC 96,Bacillus subtlis MTCC 121,three Gram-negative bacteria i.e. Pseudomonas aeruginosa MTCC 2453, Klebsiella planticola MTCC 530 and Escherichia coli MTCC 739 and one fungal strain Candida albicans MTCC 3017 according to the literature protocols [20]. The antibacterial results of prepared analogs were compared with a standard drug ampicillin whereas the antifungal potential was compared with a standard drug Amphoterecin B. Observation made for two days and ‘‘-’’ sign signifies no zone of inhibition (ZOI). In many cases,on the first day ZOI was observed but was disappeared on the next day.
Several compounds in this series (3a,3e,3g,3i,3j) showed poor activity against all bacteria. Compound 3d,was found to be exhibited good activity against B. subtilus MTCC 121 (7 mm) and moderate activity against M. luteus MTCC 2470 (12 mm). However, compounds 3k and 3l showed exclusive antibacterial activity against Gram-negative bacteria particularly P. aeruginosa MTCC 2453 with the ZOI 10 mm and 9 mm,respectively at 24 h. Compound 3f and 3 h also showed moderate activity against M. luteus MTCC 2470 (both ZOI are 13 mm at 24 h). Compound 3b and 3c were found to be active against Candida albicans MTCC 3017 with ZOI at 24 h 7 mm and 9 mm,respectively,and 3c 8 mm at 48 h. Although ampicillin is a broad spectrum antibiotic yet it is inactive against Pseudomonas and Klebsiella species at tested concentration,which may be due to some mutation.
3.2.2. Anti-inflammatory activityThe in-vivo anti-inflammatory activity was studied using the carrageenan-induced rat paw edema model [21]. The antiinflammatory activity of the test compounds was compared with a standard drug indomethacin as depicted in Table 1
|
|
Table 1 Anti-inflammatory potential of compounds 3a-l on carrageenan-induced rat paw edema (mL), % protection, relative activity and ulcer index of most active compounds to indomethacin. |
3.2.3. Ulcerogenic activity
Selected synthesized compounds (3d,3j and 3k) were also evaluated for their ulcerogenic potential relative to indomethacin as a reference drug in rats [22]. Compound 3d revealed a good ulcer index (0.92 ± 0.2) when compared with that of indomethacin (0.98 ± 0.3) while compounds 3j and 3k showed ulcer indexes of 1.86 ± 0.3 and 1.25 ± 0.2,respectively.
4. ConclusionIn summary,the present investigation describes the synthesis of novel 5-imidazolone analogs with comparable antibacterial and anti-inflammatory potencies which were characterized by suitable methods such as IR and 1H NMR. All spectral data were in accordance with assumed structures. The compounds 3c,3d,3f and 3h exhibited good to moderate activity against Gram-positive bacteria whereas compounds 3k and 3l were found active against Gram-negative bacteria. In addition,compounds 3d and 3k showed remarkable reduction in inflammation,after 4 h of carrageenan administration. The promising activity of these compounds along with the other activity data obtained during the study can also be useful in establishing the structure activity relationships and for the development of newer and more potent 5-substituted imidazolone compounds.
| [1] | M. Bhalla, P.K. Naithani, T.N. Bhalla, A.K. Saxena, K. Shanker. Novel imidazole congeners as anti-inflammatory agent. J. Indian Chem. Soc. 69 (1992) 594–595 |
| [2] | F. Suzuki, T. Kuroda, T. Tamura, New anti-inflammatory agents. 2.5-Phenyl-3Himidazo[4. 5-c]. J. Med. Chem. 35 (1992) 2863–2870 |
| [3] | S.A. El-Feky, Z.K. Abdel-Samii. Synthesis and antiinflammatory properties of some novel thiazolidinones and imidazolidinones derived from 4-(3-phenyl-4(3H)-quinazolinon-2-yl)-3-thiosemicarbazone. Pharmazie 50 (1995) 341–343 |
| [4] | M. El-Araby, A. Omar, H.H. Hassanein, H. Abdel-Ghany, A.A. El-Helby. Abdel-Rahman, design, synthesis and in vivo anti-inflammatory activities of 2,4-diaryl-5-4H-imidazolone derivatives. Molecules 17 (2012) 12262–12275 |
| [5] | W.B. Wright Jr., H.S. Brabander, R.A. Hardy Jr., A.C. Osterberg, Central nervous system depressants. I. 1-aminoalkyl-3-aryl derivatives of 2-Imidazolidinone. 1a-c2-imidazolidinethione, and tetrahydro-2(1H)-pyrimidinone1d. J. Med. Chem. 9 (1966) 852–857 |
| [6] | M. Verma, A.K. Charturvedi, A. Chaudhary, S.S. Parmar. Monoamine oxidase inhibitory and anticonvulsant properties of 1,2,4-trisubstituted 5-imidazolones. J. Pharm. Sci. 63 (1974) 1740–1744 |
| [7] | P. Upadhyay, A. Pandya, H. Parekh. Possible anticonvulsant imidazolinones synthesis and anticonvulsant activity of 1N-(Υ'-Picolinyl)-4-subsituted-benzylidene-2-methyl/phenyl-5-imidazolinone. J. Indian Chem. Soc. 68 (1991) 296–298 |
| [8] | M.A. Mesaik, K.M. Khan, S. Rahat, et al. Immunomodulatory properties of synthetic imidazolone derivatives. Lett. Drug Des. Discovery 2 (2005) 490–496 |
| [9] | E. Lunt, C.G. Newton, C. Smith, et al. Derivatives of imidazole. Ⅲ. Synthesis and pharmacological activities of nitriles, amides, and carboxylic acid derivatives of imidazo[1,2-a]pyridine. J. Med. Chem. 30 (1987) 357–366 |
| [10] | R.A. Johnson, S.M. Huong, E.S. Huang. Inhibitory effect of 4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole on HCMV DNA replication and permissive infection. Antiviral Res. 41 (1999) 101–111 |
| [11] | D.W. Robertson, E.E. Beedle, J.H. Krushinski, et al. Structure-activity relationships of arylimidazopyridine cardiotonics:discovery and inotropic activity of 2-[2-methoxy-4-(methylsulfinyl)phenyl]-1H-imidazo[4, 5-c]pyridine. J. Med. Chem. 28 (1985) 717–727 |
| [12] | P.W. Erhardt, A.A. Hagdon, D. Davey, et al. Cardiotonic agents. 5. Fragments from the heterocycle-phenyl-imidazole pharmacophore. J. Med. Chem. 32 (1989) 1173–1176 |
| [13] | K.M. Khan, U.R. Mughala, S. Khana, et al. Synthesis and antibacterial and antifungal activity of 5-substituted imidazolones. Lett. Drug Des. Discovery 6 (2009) 69–77 |
| [14] | S. Lokhandwala, N.M. Parekh. Synthesis and microbial studies of imidazolone based azetidinone analogues. Der Pharma Chem. 6 (2014) 139–142 |
| [15] | S.A. Siddiqui, S.R. Bhusare, D.V. Jarikote, R.P. Pawar, Y.B. Vibhute. New novel synthesis and antibacterial activity of 1-(substituted phenyl)-2-phenyl-4-(3'-halo 4'-hydroxy, 5'-methoxybenzylidene)-imidazole-5-one. Bull. Korean Chem. Soc. 22 (2001) 1033–1036 |
| [16] | W.B. Wright, J.H. Brabander. The rearrangement and cyclization of ethyl N-(methylaminoalkyl)carbanilates and 1,1-dimethyl-3-methylaminoalkyl-3-phenylureas. J. Org. Chem. 26 (1961) 4051–4057 |
| [17] | V. Niedbalia, I. Buettcher. Imidazole derivatives for pharmaceutical preparations. Chem. Abstr. 94 (1981) 15732 |
| [18] | A. Lingi, M. Alfonso, R. Pierluigi, et al. Derivatives of imidazole. Ⅲ. Synthesis and pharmacological activities of nitriles, amides, and carboxylic acid derivatives of imidazo[1,2-a]pyridine. J. Med. Chem. 12 (1969) 122–126 |
| [19] | E.F. Godefroi, J.T. Platje. DL-1-(.alpha.-Methylbenzyl)-2-methylimidazole-5-carboxylate esters. Synthesis and pharmacological properties. J. Med. Chem. 15 (1972) 336–337 |
| [20] | Atta-ur-Rahman, M.I. Choudhary, W.J. Thomsen. Bioassay Techniques for Drug Development, Harwood Academic Publishers. The Netherlands (2001) 15–1622 |
| [21] | C.A. Winter, E.A. Risley, G.W. Nuss. Carrageenin-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med. 111 (1962) 544–547 |
| [22] | Kumar S.G.V., D.N. Mishra. Analgesic, antiinflammatory, and ulcerogenic studies of meloxicam solid dispersion prepared with polyethylene glycol 6000. Methods Find Exp. Clin. Pharmacol. 28 (2006) 419–422 |
 2016, Vol. 27
2016, Vol. 27