b Department of Biomedical Science, College of Natural Science, Catholic University of Daegu, Gyeungsan-Si 700-702, Republic of Korea
In multi-cellular organisms,inflammation is an early,protective,homeostatic response of a host against a pathogenic challenge [1] and is indicative of either acute or chronic inflammation. In normal conditions,this process is automatically regulated by the limiting expression levels of pro-inflammatory cytokines,but under pathological conditions,macrophage stimulation leads to an increase of nitric oxide (NO) production. NO,short-lived free radical,can regulate various physiological functions in the cardiovascular,nervous and immune system [2]. Its endogenous secretion from L-arginine is catalyzed by a family of nitric oxide synthase (NOS) enzymes viz. neuronal NOS (nNOS),endothelial NOS (eNOS) and inducible NOS (iNOS). The first two are constitutively expressed and can generate physiologically vital amounts of NO involved chiefly in nerve function and blood regulation whereas the later one (i.e. iNOS) produces larger amounts (nano molar) in response to various proinflammatory stimuli. Overproduction of NO causes cell damage because of its highly reactive nature. Therefore,effective control of NO accumulation by iNOS inhibition represents a beneficial therapeutic strategy.
Nonsteroidal anti-inflammatory drugs (NSAIDs) and classical steroidal anti-inflammatory drugs (SAIDs) are currently used to treat acute inflammation. Treatment of chronic inflammation with these SAIDs and NSAIDs is not absolutely successful due to unexpected side effects associated with these developed compounds. Hence,there is a need for the identification and development of safe,effective and novel anti-inflammatory agents.
Bacterial lipopolysaccharides (LPSs) are the major outer surface membrane components present in almost all Gram-negative bacteria and can induce the production of inflammatory mediators including iNOS in diverse eukaryotic species ranging from insects to humans [3]. Therefore,reducing the expression levels of LPSinducible inflammatory mediators is a promising method to attenuatea varietyofdisorders derived frominflammationtriggered by activated macrophages. RAW 264.7 is a murine macrophage cell line which has been established as an excellent model to screen antiinflammatory activity of bioactive compounds.
Chalcones,as members of the flavonoid family,are a distinguished class of naturally occurring,bioactive compounds with 1,3-diaryl-2-propen-1-one skeleton. They are abundantly present in edible plants and are important precursors in the biosynthesis of flavonoids and isoflavonoids [4]. Primitive therapeutic applications of these plant-related,secondary metabolites can be associated with the thousand-year old use of plants and herbs for the treatment of different medical disorders. These small and nonchiral chemical templates possess a conjugated double bond and an entirely delocalized π-electron system on both benzene rings which gives the compounds non-linear optical properties [5]. Recently,chalcones have been a subject of great interest around the globe in view of their availability in nature,effortless synthesis,accessible structural modifications and multifarious biological activities. Various natural and non-natural chalcones have been investigated as anti-inflammatory [6],antioxidant [7],anticancer [8],antiprotozoal [9],antimicrobial [10],antiviral [11],antibacterial [12],antihyperglycemic [13],antiplatelet aggregation [14],antiangiogenic [15],antiulcerative [16],antitubercular [17],and antiplasmodial [18] agents. They have also shown inhibitory effects on several enzymes [19].
Continuing our interest on the synthesis and biological evaluation of chalcones as anti-inflammatory agents [20],herein we describe the synthesis of natural prenylated and pyranochalcones using a Claisen-Schmidt condensation as a key step and present the assessment of their anti-inflammatory effects.
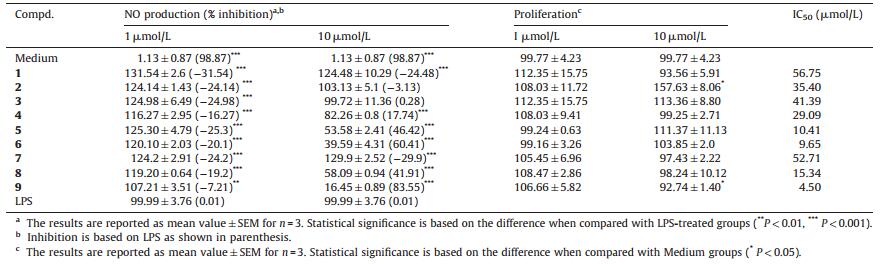
Natural prenylated chalcones under the current study viz. kanzonol C (1) [21],stipulin (2) [22],crotaorixin (3) [23],medicagenin (4) [24],licoagrochalcone A (5) [25] and abyssinone D (6) [26] were isolated from Glycyrrhiza eurycarpa,Dalbergia stipulacea,Crotalaria orixensis,Crotalaria medicaginea,Glycyrrhiza glabra,and Erythrina abyssinica,respectively (Fig. 1). Pyranochalcones include in this investigation are paratocarpin C (7) [27],anthyllisone (8) [28] and 3-O-methylabyssinone A (9) [29] which were isolated from Paratocarpus Venezosa Zoll,Anthyllis hermanniae and Lonchocarpus nicou,respectively (Fig. 1). Synthesis of these interesting compounds was not yet been reported,except for compounds 4 [30] and 5 [31].

|
Download:
|
| Fig. 1. Structures of naturally occurring prenylated and pyranochalcones (1-9). | |
2. Experimental
All chemicals were obtained from commercial suppliers and were used without further purification unless noted otherwise. All solvents used for reactions were freshly distilled from proper dehydrating agents under nitrogen gas. All solvents used for chromatography were purchased and directly used without further purification. The 1H NMR spectra were recorded at Varian Mercury-300 MHz FT-NMR and 75 MHz for 13C NMR,with the chemical shift (δ) reported in parts per million (ppm) downfield relative to TMS and the coupling constants (J) quoted in Hz. CDCl3/ CD3OD/CD3COCD3 was used as solvent and an internal standard. Mass spectra were recorded using Agilent-5977E spectrometer. Melting points were measured on a MEL-TEMP Ⅱ apparatus and were uncorrected. Thin-layer chromatography (TLC) was performed on DC-Plastikfolien 60,F254 (Merck,layer thickness 0.2 mm) plastic-backed silica gel plates and visualized by UV light (254 nm) or staining with p-anisaldehyde. Chromatographic purification was carried out using Kieselgel 60 (60-120 mesh,Merck).
2.1. General procedure for Claisen-Schmidt condensation reactionTo a stirred solution of acetophenone (0.25 mmol) and aromatic aldehyde (0.3 mmol,1.2 equiv.) in MeOH (2 mL) and H2O (1 mL) was added KOH (0.309 g,5.5 mmol,22 equiv.) and the mixture was stirred at room temperature for 72 h. After completion of the reaction,H2O (25 mL) was added and extracted with EtOAc (3 × 25 mL). The combined organic layer was washed with brine (2 × 40 mL),dried over anhydrous Na2SO4 and concentrated in vacuo. The crude was purified by column chromatography (EtOAc/hexane = 1/20 to 1/5) to obtain the allyl protected chalcone.
2.2. General procedure for allyl group deprotectionTo a stirred solution of di- or tri-allyloxy chalcone (0.15 mmol) in anhydrous MeOH (2.5 mL) were added K2CO3 (0.124 g,0.9 mmol,6 equiv.) and Pd(PPh3)4 (2 mmol%) at room temperature and degassed for 2 min. The reaction mixture was stirred at 60 ℃ for 1-1.5 h. After completion of the reaction,solvent was removed in vacuo. H2O (15 mL) was added to the crude,neutralized with slow addition of 1 mol/L HCl (1.5 mL) at 0 ℃ and then extracted with EtOAc (3 × 30 mL). The combined organic layer was washed with brine (2 × 40 mL),dried over anhydrous Na2SO4 and concentrated in vacuo. The crude was purified by column chromatography (EtOAc/hexane = 1/5 to 1/1,v/v) to obtain the pure chalcone.
Physical and spectroscopic characterization data of the compounds described in this article were given in Supporting information.
3. Results and discussion 3.1. ChemistryOur approach for the synthesis of the chalcones 1-9 is outlined in Schemes 1-4. The synthesis commenced with the prenylation of 2,4-dihydroxyacetophenone (10) following the literature procedure [31b] (Scheme 1).
|
Download:
|
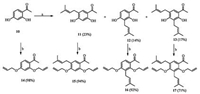
| Scheme. 1. Synthesis of prenyl substituted acetophenones. Reagents and conditions: (a) 2-methyl-but-3-en-2-ol, BF3.Et2O, 1,4-dioxane, r.t., 1 h. (b) allyl bromide, Cs2CO3/NaI, DMF, 60 ℃, 5 h. | |
Treatment of compound 10 with BF3.Et2O followed by 2- methyl-but-3-en-2-ol addition at room temperature afforded compounds 11-13. Subsequently,allyl protection of 10 along with the prenylated acetophenones 11-13 was accomplished with Cs2CO3/NaI and allyl bromide in DMF at 60 ℃ and the resulting products 14-17 were each obtained in high yields.
Next,4-hydroxybenzaldehyde (18) and vanillin (19) were transformed to their prenyl derivatives (Scheme 2). Treatment of 18 and 19 with 3,3-dimethylallyl bromide using 1 mol/L NaOH afforded the aldehydes 20 and 21,respectively. Later,aldehydes 18-21 were reacted with allyl bromide in the presence of Cs2CO3/NaI to generate the allyl protected aldehydes 22-25 in high yields,respectively.

|
Download:
|
| Scheme. 2. Synthesis of prenyl substituted benzaldehydes. Reagents and conditions: (a) 3,3-dimethylallyl bromide, 1 mol/L NaOH, 0 ℃—r.t., 2 h. (b) allyl bromide, Cs2CO3/NaI, DMF, 60 ℃, 2 h. | |
Aldehyde part of pyranochalcones 7-9 was obtained from aldehydes 18,20 and 19 respectively (Scheme 3). Reaction of aldehydes 18-20 with 3-chloro-3-methyl-1-butyne using K2CO3/ KI and catalytic CuI in acetone under reflux gave compounds 26-28 which were subsequently converted to compounds 29-31,respectively.

|
Download:
|
| Scheme. 3. Synthesis of substituted 2,2-dimethyl-2H-chromene-6-carbaldehydes. Reagents and conditions: (a) 3-chloro-3-methyl-1-butyne, K2CO3/KI, acetone, reflux, 4.5 h. (b) N, N-diethylaniline, sealed tube, 210 ℃, 1.5 h. | |
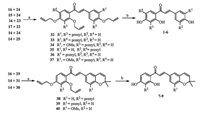
Keeping the allyl protected acetophenones (14-17) and aromatic aldehydes (22-25 and 29-31) in hand,next we executed the Claisen-Schmidt condensation (Scheme 4). Treatment of the suitable acetophenone with 1.2 equiv. of aromatic aldehyde using excess KOH in MeOH:H2O (2:1) at room temperature produced the allyl protected chalcones (32-40) in good to high yields. Finally,allyl group deprotection was achieved using K2CO3 and catalytic Pd(PPh3)4 (2 mol%) in anhydrous MeOH at 60o C to furnish the desired natural prenylated and pyranochalcones 1-9. All the products 1-9 were structurally confirmed by their spectral (1H NMR & 13C NMR and MS) data.
|
Download:
|
| Scheme. 4. Synthesis of naturally occurring prenylated and pyranochalcones. Reagents and conditions: (a) KOH, MeOH/H2O (2:1), r.t., 72 h, 58%-90%. (b) K2CO3, Pd(PPh3)4, MeOH, 60 ℃, 1-1.5 h, 51%-89%. | |
3.2. Pharmacology
To examine the anti-inflammatory effect of the synthesized chalcones 1-9,we selected an in vitro model with murine RAW 264.7 macrophage cell line,which is an established model for anti-inflammatory drug screening. We assayed the ability of compounds 1-9 to decrease NO release after LPS stimulation. The RAW 264.7 cells were incubated with LPS and 1 μmol/L & 10 μmol/L concentrations of compounds 1-9 for 24 h. Later,culture media were harvested and the amount of NO was measured. Compounds 1-4 and 7 exhibited weak,or no suppression of NO production at 10 μmol/L level (Table 1). On the other hand,chalcones 5,6,8 and 9 were shown to exhibit a moderate to good level of activity.
|
|
Table 1 Anti-inflammatory activities of naturally occurring prenylated and pyranochalcones 1-9. |
Next,cell viability was analyzed to check whether the inhibitory effect was due to cytotoxicity. All of the prepared chalcones displayed no toxic effects,except compound 9,which showed a slight cytotoxicity at 10 μmol/L level (Table 1). The IC50 values of these natural chalcones 1-9 were evaluated by using GraphPad Prism 4.0 software and the values were 56.75,35.40,41.39,29.09,10.41,9.65,52.71,15.34 and 4.50 μmol/L,respectively. Based upon the results,it can be concluded that chalcones bearing the prenyl group only on ring B (aldehyde part),i.e.,5,6 and 8 are fruitful to show good anti-inflammatory activity by effective inhibition of NO production with no cytotoxicity.
4. ConclusionWe have developed a simple and efficient approach for the synthesis of naturally occurring prenylated and pyranochalcones 1-9 using the Claisen-Schmidt condensation as a key step. Later,their anti-inflammatory effects were evaluated in lipopolysaccharides (LPSs)-stimulated RAW-264.7 macrophages. Of the chalcones prepared in this study,compounds 5,6 and 8 showed remarkable activities with no cytotoxicity. Compound 9 (IC50 = 4.5 μmol/L) exhibited maximum (83.6%) nitric oxide (NO) inhibition,but showed a slight cytotoxicity. The results revealed that the chalcones bearing the prenyl group at 3- and/or 5-position on ring A (acetophenone part),i.e.,1-4 and 7 showed weak or no inhibition activities,whereas chalcones holding prenyl group only on ring B (aldehyde part),i.e.,5,6 and 8 showed significant activities on the production of inflammatory mediated NO with no cytotoxicity.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2016.01.043.
| [1] | J. Quintans. Immunity and inflammation:the cosmic view. Immunol. Cell Biol. 72 (1994) 262–264 |
| [2] | S. Moncada, R.M. Palmer, E.A. Higgs. Nitric oxide:physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 43 (1991) 109–142 |
| [3] | B. Hinz, K. Brune. Cyclooxygenase-2-10 years later. J. Pharmacol. Exp. Ther. 300 (2002) 367–375 |
| [4] | D.I. Batovska, I.T. Todorova. Trends in utilization of the pharmacological potential of chalcones. Curr. Clin. Pharmcol. 5 (2010) 1–29 |
| [5] | (a) A.K. Singh, G. Saxena, R. Prasad, A. Kumar, Synthesis, characterization and calculated non-linear optical properties of two new chalcones, J. Mol. Struct. 1017(2012) 26-31; (b) E.D. D'silva, G.K. Podagatlapalli, S.V. Rao, et al., New, high efficiency nonlinear optical chalcone co-crystal and structure-property relationship, Cryst. Growth Des. 11(2011) 5362-5369. |
| [6] | S.J. Won, C.T. Liu, L.T. Tsao, et al. Synthetic chalcones as potential anti-inflammatory and cancer chemopreventive agents. Eur. J. Med. Chem. 40 (2005) 103–112 |
| [7] | J.F. Stevens, C.L. Miranda, B. Frei, D.R. Buhler. Inhibition of peroxynitrite-mediated LDL oxidation by prenylated flavonoids:the α,β-unsaturated keto functionality of 2'-hydroxychalcones as a novel antioxidant pharmacophore. Chem. Res. Toxicol. 16 (2003) 1277–1286 |
| [8] | D.K. Mahapatra, S.K. Bharti, V. Asati. Anti-cancer chalcones:structural and molecular target perspectives. Eur. J. Med. Chem. 98 (2015) 69–114 |
| [9] | F. Lunardi, M. Guzela, A.T. Rodrigues, et al. Trypanocidal and leishmanicidal properties of substitution containing chalcones. Antimicrob. Agents Chemother. 47 (2003) 1449–1451 |
| [10] | M. Ritter, R.M. Martins, D. Dias, M.P. Pereira. Recent advances on the synthesis of chalcones with antimicrobial activities:a brief review. Lett. Org. Chem. 11 (2014) 498–508 |
| [11] | J.Y. Park, H.J. Jeong, Y.M. Kim, et al. Characteristic of alkylated chalcones from Angelica keiskei on influenza virus neuraminidase inhibition. Bioorg. Med. Chem. Lett. 21 (2011) 5602–5604 |
| [12] | S.F. Nielsen, T. Boesen, M. Larsen, et al. Antibacterial chalcones-bioisosteric replacement of the 4'-hydroxy group. Bioorg. Med. Chem. 12 (2004) 3047–3054 |
| [13] | C.T. Hsieh, T.J. Hsieh, M. El-Shazly, et al. Synthesis of chalcone derivatives as potential anti-diabetic agents. Bioorg. Med. Chem. Lett. 22 (2012) 3912–3915 |
| [14] | L.M. Zhao, H.S. Jin, L.P. Sun, et al. Synthesis and evaluation of antiplatelet activity of trihydroxychalcone derivatives. Bioorg. Med. Chem. Lett. 15 (2005) 5027–5029 |
| [15] | L. Varinska, M. van Wijhe, M. Belleri, et al. Anti-angiogenic activity of the flavonoid precursor 4-hydroxychalcone. Eur. J. Pharmacol. 691 (2012) 125–133 |
| [16] | K.V. Shashidhara, S.R. Avula, V. Mishra, et al. Identification of quinoline-chalcone hybrids as potential antiulcer agents. Eur. J. Med. Chem. 89 (2015) 638–653 |
| [17] | F. Macaev, V. Boldescu, S. Pogrebnoi, G. Duca. Chalcone scaffold based antimycobacterial agents. Med. Chem. 4 (2014) 487–493 |
| [18] | M. Larsen, H. Kromann, A. Kharazmi, S.F. Nielsen. Conformationally restricted anti-plasmodial chalcones. Bioorg. Med. Chem. Lett. 15 (2005) 4858–4861 |
| [19] | (a) O. Nerya, R. Musa, S. Khatib, et al., Chalcones as potent tyrosinase inhibitors:the effect of hydroxyl positions and numbers, Phytochemistry 65(2004) 1389-1395; (b) S. Iwata, N. Nagata, A. Omae, et al., Inhibitory effect of chalcone derivativess on recombinant human aldose reductase, Biol. Pharm. Bull. 22(1999) 323-325. |
| [20] | (a) S.J. Kim, C.G. Kim, S.R. Yun, et al., Synthesis of licochalcone analogues with increased anti-inflammatory activity, Bioorg. Med. Chem. Lett. 24(2014) 181-185; (b) J.H. Jeon, M.R. Kim, J.G. Jun, Concise synthesis of licochalcone A through water-accelerated[3,3]-sigmatropic rearrangement of an aryl prenyl ether, Synthesis 43(2011) 370-376. |
| [21] | T. Fukai, J. Nishizawa, T. Nomura. Five isoprenoid-substituted flavonoids from Glycyrrhiza eurycarpa. Phytochemistry 35 (1994) 515–519 |
| [22] | P. Bhatt, R. Dayal. Stipulin, a prenylated chalcone from Dalbergia stipulacea. Phytochemistry 31 (1992) 719–721 |
| [23] | T. Narender, S.K. Tanvir, M.S. Rao, et al. Prenylated chalcones isolated from Crotalaria genus inhibits in vitro growth of the human malaria parasite Plasmodium falciparum. Bioorg. Med. Chem. Lett. 15 (2005) 2453–2455 |
| [24] | G.V.R. Rao, P.S. Rao, K.R. Raju. A prenylated chalcone from Crotalaria medicaginea. Phytochemistry 26 (1987) 2866–2868 |
| [25] | Y. Asada, W. Li, T. Yoshikawa. Isoprenylated flavonoids from hairy root cultures of Glycyrrhiza glabra. Phytochemistry 47 (1998) 389–392 |
| [26] | L. Cui, P.T. Thuong, H.S. Lee, et al. Four new chalcones from Erythrina abyssinica. Planta Med. 74 (2008) 422–426 |
| [27] | Y. Hano, N. Itoh, A. Hanaoka, et al. Paratocarpins A-E, five new isoprenoidsubstituted chalcones from Paratocarpus venenosa Zoll. Heterocycles 41 (1995) 191–198 |
| [28] | L. Pistelli, K. Spera, G. Flamini, et al. Isoflavonoids and chalcones from Anthyllis hermanniae. Phytochemistry 42 (1996) 1455–1458 |
| [29] | M.A. Lawson, M. Kaouadji, A.J. Chulia. A single chalcone and additional rotenoids from Lonchocarpus nicou. Tetrahedron Lett. 51 (2010) 6116–6119 |
| [30] | (a) G.V. Rao, B.N. Swamy, V. Chandregowda, G.C. Reddy, Synthesis of (±)-abyssinone I and related compounds:their anti-oxidant and cytotoxic activities, Eur. J. Med. Chem. 44(2009) 2239-2245; (b) A. Maiti, M. Cuendet, V.L. Croy, et al., Synthesis and biological evaluation of (±)-abyssinone Ⅱ and its analogues as aromatase inhibitors for chemoprevention of breast cancer, J. Med. Chem. 50(2007) 2799-2806. |
| [31] | (a) H.-M. Wang, L. Zhang, J. Liu, et al., Synthesis and anti-cancer activity evaluation of novel prenylated and geranylated chalcone natural products and their analogs, Eur. J. Med. Chem. 92(2015) 439-448; (b) N. Tadigoppula, V. Korthikunta, S. Gupta, et al., Synthesis and insight into the structure-activity relationships of chalcones as antimalarial agents, J. Med. Chem. 56(2013) 31-45. |
 2016, Vol. 27
2016, Vol. 27