b Shanxi Province Key Laboratory of Functional Nanocomposites, North University of China, Taiyuan 030051, China ;
c Department of Materials Science and Engineering, University of Texas at Arlington, Arlington, TX 76019, USA
In recent years,silver nanomaterials have received great interest due to their potential applications in catalysis [1],biology [2],electronics [3, 4],and optoelectronics [5, 6]. A great deal of effort has been put into the research of the synthesis of silver nanostructures. Xia’s group [7, 8, 9] has explored the polyol synthesis method to produce controllable Ag nanostructures. Based on numerous studies,they have concluded that the final shape of a nanocrystal is closely related to the internal structure of the seed and the capping agent. They also found that the oxidative etching of Cl- can result in the dissolution of twinned seeds,and that Bronly can selectively remove the multiply twinned seeds. ManzanoRamírez [10] has also confirmed that silver nanowires could be obtained with short-chain PVP as the capping agent. In this process,it was found that a longer stirring time could lead to the formation of acicular particles. Shaban prepared Ag nanoparticles by a green and rapid method using sunlight and cationic surfactants [11]. Also some green synthesis methods have been developed to produce Ag nanoparticles,such as synthesis with the aqueous extracts of Enteromorpha flexuosa as the reductant [12].
Here we study the effect of mechanical agitation,either stirring or sonication,on the morphology of nanoparticles when synthesizing Ag nanoparticles by reducing AgNO3 with AA as the reductant and PVP as the capping agent. With vigorous stirring,flower-like Ag nanoparticles formed. However,trace amounts of Cl- could drastically change the nanoparticle morphology. When the sonication was used for the agitation,only dispersed nanoparticles were produced. Flower-like Ag nanoparticles have a highly roughed surface which gives rise to a good performance as the substrate for SERS.
2. ExperimentalThe flower-like Ag nanoparticles were synthesized by chemical reduction of AgNO3,with AA as the reductant. In a typical synthesis process,AgNO3 aqueous solution (0.06 mol/L,9 mL) and PVP (0.06 mol/L,9 mL) aqueous solution were added into beaker with magnetic stirring at room temperature. The PVP concentration was calculated in terms of the repeating units. After 30 min magnetic stirring,AA (0.2 mol/L,2 mL) was quickly injected into the mixture. Immediately,the color of solution turned into dark grey,indicating the formation of a large quantity of Ag nanoparticles. After 30 min magnetic stirring,the reaction liquid was centrifuged at 13,000 rpm for 15 min,followed by alternately washing with water and ethanol 3 times. Finally,Ag nanoparticles were dispersed in 5 mL ethanol.
UV-vis spectra was recorded on a Unico UV4802 UV/vis spectrometer. Scanning electron microscopy (SEM) analysis was performed on Tescan MIRA 3LMH scanning electron microscope. The crystal structures of the silver nanoparticles were analyzed by the powder X-ray diffraction (XRD) with Cu-Kα source (Siemens D500) with patterns recorded in the range of 30-808 (2θ). Raman spectra was obtained from Raman spectroscopy HR800 (Jobin-yvon).
3. Results and discussionFig. 1 shows the morphology ofthe products. Itclearly showsthat mechanical agitation can cause very different morphologies. When the solution was vigorously stirred using a magnetic stirrer,the flower-like Ag nanoparticles were produced (Fig. 1a-C). With the concentrations of AgNO3 and PVP increased from 0.06 mol/L to 0.1 mol/L,the size offlower-likeAg nanoparticles are increased from 500 nm to 1000 nm. The petal of flower-like nanoparticles gradually changed from nanorods to nanosheets. The transformation can be clearly found from Fig. 1A,B and C.It is well known that the growth of flower-like silver nanoparticles can be attributed to the outgrowth process [13, 14]. After the quick injection of AA,the color of the solution was immediately turned into dark grey,indicating a rapid reaction. With vigorous stirring,the transportation of the reactants became faster to raise the growth rate of the silver nanostructures [13]. As the increasing of concentration,the rate of production and growth of seed crystals increases. The number of flake-like Ag nanoparticles has significantly increased,which might be explained from the fact that a higher rate is conducive to produce seeds with stacking faults.
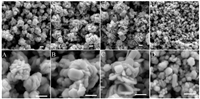
|
Download:
|
| Fig. 1. SEM images of Ag nanoparticles obtained by changing the concentration of AgNO3 (PVP kept the same with AgNO3) in reaction solution and the vigorous stirring reacting. (a) and (A) 0.06 mol/L, (b) and (B) 0.08 mol/L, (c) and (C) 0.1 mol/L; (d) and (D) 0.06 mol/L (mixing the reaction solution in ultrasonic field). All scale bars are 300 nm. | |
When ultrasonic agitation was used,dispersed nanoparticles with a wide size distribution can be observed (Fig. 1d and D). Mechanical stirring can offer shear forces and significantly accelerate the transfer of mass [15],but ultrasonic agitation accelerate the Brownian movement of Ag colloid. Ultrasound radiation resulted in the homogenously dispersion of PVP in solution and the production of stable spherical micelles [16]. Spherical nanoparticles could be produced by the reduction of silver ions inside these micellar templates. In addition,these dispersed nanoparticles may result from the dissociation from the flower-like silver nanoparticles [17]. Moreover,mass transport with stirring is favorable for isotropic and compact growth,which may result in the aggregation of Ag nanoparticles from some smaller nanoparticles [10].
Fig. 2 shows the XRD patterns of these Ag nanoparticles. It indicates the formation of highly pure silver nanoparticles with perfect crystallization. The intensity ratio of the (1 1 1) to (2 0 0) peak is higher than that of the standard card of silver (PDF#87-0720 file),which indicates that these Ag nanoparticles are growing along the (1 1 1) direction. This is observed specially in sample C. In this case,the observed (1 1 1)/(2 0 0) intensity ratio (3.01) is higher than standard value (2.5). No obvious differences can be found among these XRD patterns,which suggests that the reactant concentration,magnetic stirring and ultrasonic oscillations perform no effects on silver nanocrystal.

|
Download:
|
| Fig. 2. XRD patterns of Ag nanoparticles obtained by changing the concentration of AgNO3 (PVP kept the same with AgNO3) in reacting solution and vigorous stirring the reacting solution. (a) 0.06 mol/L, (b) 0.08 mol/L, (c) 0.1 mol/L; (d) 0.06 mol/L (mixing the reacting solution in ultrasonic field). | |
Fig. 3a-c depict the SEM images of the silver nanoparticles synthesized with addition of trace amounts of sodium chloride (NaCl,4.5 mmol/L) at different stage 0.5,24,and even 48 h. The reaction conditions are the same for the particles shown in Fig. 1a. No flower-like or flake-like Ag nanoparticles were observed,but only well-dispersed silver nanoparticles (Fig. 3a-c). It can be attributed to the presence of Cl-. At the initial stage of nucleation,the concentration of free Ag+ is decreased as the formation of AgCl colloids. Conversely,with the reduction of Ag+,AgCl colloid subsequently releases Ag+ to the solution. The absorption and release of Ag+ give rise to a stable reaction of reduction of silver nitrate [18]. Notably,with the increase of reaction time,the average sizes of Ag nanoparticles decrease,which is in accordance with the blue shift from 445 nm to 405 nm in UV-vis absorption spectra (Fig. 3d). The Cl- can remove the defected seeds from the solution because of the oxidative etching. Hence,flower-like and flake-like Ag nanoparticles disappeared,finally. The wide absorption peak in UV-vis absorption spectrum indicates the products of Ag nanoparticles have a wide particle size distribution.

|
Download:
|
| Fig. 3. (a)-(c) SEM micrographs of Ag nanostructures synthesized in the presence of NaCl at different stages 0.5, 24 and 48 h, respectively. All scale bars are 250 nm. (d) UV-vis spectra of eleven samples taken from the same reaction between 0.5 h and 48 h. | |
To evaluate the ability of flower-like Ag nanoparticle as SERS substrates,R6G was chosen as the probe molecule. Fig. 4 shows the Raman spectra of the R6G solution with different concentrations obtained from flower-like Ag nanoparticles. These flower-like Ag nanoparticles were synthesized by the same condition as Fig. 1a. Strong Raman peaks can be clearly observed and can be indexed as the typical R6G Raman spectra as reported in the literature [19]. Compared to curve 5,a greater enhancement of Raman signals can be found in curve 1. There is no distinct signal can be found in curve 6. The Raman peaks of R6G can also be found clearly even at a much lower concentration of 10-7 mol/L,which indicates the flower-like Ag nanoparticle can act as SERS substrates for ultra sensitive detection. The enhancement factor (EF) can be rough calculated trough the following equation: EF = (ISERS/INR) × (CNR/ CSERS),where ISERS and INR are the band intensity of the selected band at 1364 cm-1 obtained by SERS and corresponding band intensity of the bulk solution,respectively. CSERS and CNR are the corresponding concentrations of R6G in the SERS and bulk solution. The EF for SERS detection of R6G in our experiment was estimated to be 1.3 × 105. The good performance of the flower-like Ag nanoparticle for SERS likely arises from the gaps among these nanoparticles,which act as hot spots with an extremely high SERS enhancement factor.

|
Download:
|
| Fig. 4. Raman spectrum of solid R6G obtained from silicon wafer (10-2 mol/L, curve 5) and SERS spectra of R6G at different concentrations: curves 1-4 are 10-4, 10-5, 10-6 and 10-7 mol/L R6G obtained from flower-like Ag nanoparticles, respectively. Curve 6 is the Raman spectrum of flower-like Ag nanoparticles. | |
4. Conclusion
In conclusion,by controlling the solution component,concentration,and mechanical agitation methods (magnetic stir or ultrasonic sonication),we obtained silver nanoparticles with different and distinct morphologies. The flower-like Ag nanoparticles were synthesized by reducing AgNO3 using AA as reductant and PVP as the capping agent under vigorous stirring condition. Such particles are the aggregates of smaller nanorods and nanoplates. However,the flower-like Ag nanoparticles would be replaced by well-dispersed nanoparticles if the reagent was in ultrasonic field or by adding trace amounts of NaCl. Flower-like Ag nanoparticles were tested as substrates for the Surface-Enhanced Raman Scattering,showing high sensitivity for detecting Rhodamine 6G (R6G) at a concentration as low as 10-7 mol/L. This likely arises from the gaps among these nanoparticles which act as hot spots with extremely high SERS enhancement factor.
| [1] | X. Liu, Z.Q. Liu, S.X. Hao, W. Chu. Facile fabrication of well-dispersed silver nanoparticles loading on TiO2 nanotube arrays by electrodeposition. Mater. Lett. 88 (2012) 66–68 |
| [2] | P.J. Rivero, A. Urrutia, J. Goicoechea, et al. An antibacterial submicron fiber mat with in situ synthesized silver nanoparticles. J. Appl. Polym. Sci. 126 (2012) 1228–1235 |
| [3] | S.X. Ouyang, J.H. Ye. β-AgAl1-xGaxO2 Solid-solution photocatalysts:continuous modulation of electronic structure toward high-performance visible-light photoactivity. J. Am. Chem. Soc. 133 (2011) 7757–7763 |
| [4] | J. Li, D. Zhang, J.B. Guo, J. Wei. Electrochemical behavior and specific capacitance of polyaniline/silver nanoparticle/multi-walled carbon nanotube composites. Chin. J. Chem. Phys. 27 (2014) 718–724 |
| [5] | L.M. Chen, Y.N. Liu. Ag-nanoparticle-modified single Ag nanowire for detection of melamine by surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 43 (2012) 986–991 |
| [6] | Y. Liu, L.Q. Huang, J. Wang, et al. Fabrication of silver ordered nanoarrays SERSactive substrates and their applications in bladder cancer cells detection. Spectrosc. Spect. Anal. 32 (2012) 386–390 |
| [7] | Y.N. Xia, Y.J. Xiong, B. Lim, et al. Shape-controlled synthesis of metal nanocrystals:simple chemistry meets complex physics?. Angew. Chem. Int. Ed. Engl 48 (2008) 60–103 |
| [8] | B. Wiley, Y.G. Sun, Y.N. Xia. Synthesis of silver nanostructures with controlled shapes and properties. Acc. Chem. Res. 40 (2007) 1067–1076 |
| [9] | C.M. Cobley, S.E. Skrabalak, D.J. Campbell, Y.N. Xia. Shape-controlled synthesis of silver nanoparticles for plasmonic and sensing applications. Plasmonics 4 (2009) 171–179 |
| [10] | A. Gómez-Acosta, A. Manzano-Ramírez, E.J. López-Naranjo, et al. Silver nanostructure dependence on the stirring-time in a high-yield polyol synthesis using a short-chain PVP. Mater. Lett. 138 (2015) 167–170 |
| [11] | S.M. Shaban, I. Aiad, M.M. El-Sukkary, E.A. Soliman, M.Y. El-Awady. Preparation of capped silver nanoparticles using sunlight and cationic surfactants and their biological activity. Chin. Chem. Lett. 26 (2015) 1415–1420 |
| [12] | M. Yousefzadi, Z. Rahimi, V. Ghafori. The green synthesis, characterization and antimicrobial activities of silver nanoparticles synthesized from green alga Enteromorpha flexuosa (wulfen). J. Agardh, Mater. Lett 137 (2014) 1–4 |
| [13] | T. Liu, D.S. Li, D.R. Yang, M.H. Jiang. Fabrication of flower-like silver structures through anisotropic growth. Langmuir 27 (2011) 6211–6217 |
| [14] | L.J. Hong, Q. Li, H. Lin, Y. Li. Synthesis of flower-like silver nanoarchitectures at room temperature. Mater. Res. Bull. 44 (2009) 1201–1204 |
| [15] | S. Coskun, B. Aksoy, H.E. Unalan. Polyol synthesis of silver nanowires:an extensive parametric study. Cryst. Growth Des. 11 (2011) 4963–4969 |
| [16] | J. Moghimi-Rad, T.D. Isfahani, I. Hadi, et al. Shape-controlled synthesis of silver particles by surfactant self-assembly under ultrasound radiation. Appl. Nanosci. 1 (2011) 27–35 |
| [17] | K.S. Suslick, D.A. Hammerton, R.E. Cline. Sonochemical hot spot. J. Am. Chem. Soc 108 (1986) 5641–5642 |
| [18] | D.P. Chen, X.L. Qiao, X.L. Qiu, J.G. Chen, R.Z. Jiang. Large-scale synthesis of silver nanowires via a solvothermal method. J. Mater. Sci. 22 (2011) 6–13 |
| [19] | J.J. Xi, Y.H. Ni, A.M. Liu. Versatile Ag dendrites:simple galvanostatic deposition and applications. New J. Chem. 38 (2014) 1738–1742 |
 2016, Vol. 27
2016, Vol. 27 


