b Key Laboratory of Green Chemistry of Sichuan Institutes of Higher Education, Zigong 643000, China
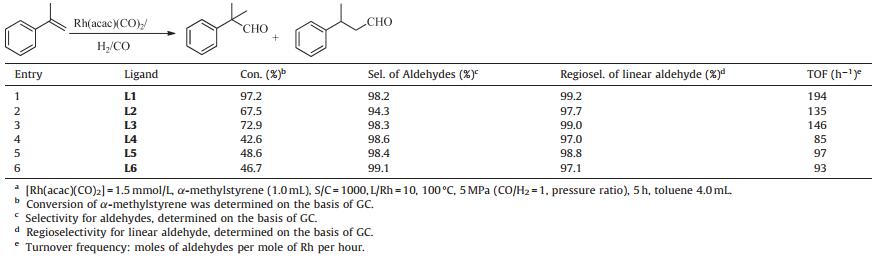
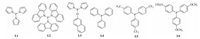
The rhodium-catalyzed hydroformylation of a-methylstyrene is of great interest, because the corresponding aldehydes are important intermediates for the production of spices. Due to the bulky phenyl and the adjacent methyl groups, the hydroformylation proceeds quite slowly. Therefore, the development of an effective catalyst is a necessary challenge. Keoken and co-workers reported that tri(2, 4-di-tert-butylphenyl) phosphite modified rhodium could catalyze the hydroformylation of α-methylstyrene with a TOF of 130 h-1 in supercritical CO2 [1]. A cationic rhodium complex containing a bis(dioxaphospholane) ligand exhibited high regioselectivity in the hydroformylation of α-methylstyrene; however, the TOF value was less than 2.5 h-1 [2]. The above results are not satisfactory; the main problem may lie in the steric hindrance of α-methylstyrene caused by the phenyl and methyl groups on C-1. It is well known that the ligand plays a vital role in the performance of a catalyst via its steric and electronic properties. In this study, in order to find a highly active catalyst for α-methylstyrene hydroformylation, six ligands with different steric and electronic properties, tris(N-pyrrolyl)phosphine (L1), tris(9-carbazolyl)phosphine (L2), naphthalenyldipyrrolylphosphorusamidite (L3), triphenylphosphine (L4), tris(4-trifluoromethylphenyl)phosphine (L5), and tris(4-methoxyphenyl) phosphine (L6) were investigated with Rh(acac)(CO)2 as the catalyst precursor (Fig. 1), and the reaction parameters were also optimized.
|
Download:
|
| Fig. 1. Structures of ligands L1-L6. | |
2. Experimental
α-Methylstyrene (>99%) was redistilled prior to use and all other reagents were of analytical grade and used as received. Rh(acac)(CO)2 and ligands L1, L2, L3, L5, and L6 were prepared according to the literature [3, 4, 5, 6], and their structures were confirmed by 1H NMR, 13C NMR and 31P NMR measurements. All the hydroformylation reactions were carried out in a stainless steel autoclave of 60 μL with a magnetic stirrer. A toluene solution of Rh(acac)(CO)2, ligand, and a-methylstyrene were added to the autoclave, which was subsequently evacuated and purged three times with 1 MPa of syngas (H2/CO = 1:1). The autoclave was then pressurized with syngas and stirred under the specific reaction conditions. After the reaction was completed, the vessel was cooled to room temperature before excess syngas was carefully released. The products were then analyzed on Agilent 6890 N gas chromatograph with a capillary column SE-30 (30 m × 0.25 mm) and identified by GC-MS and NMR.
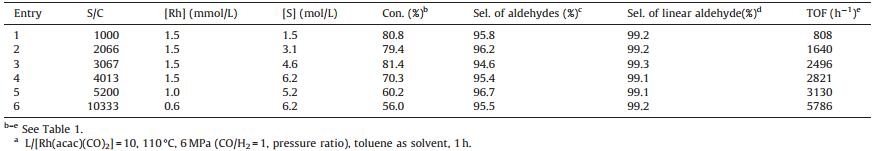
3. Results and discussionFrom the outset, six different ligands (Fig. 1) were studied in the Rh-catalyzed hydroformylation of a-methylstyrene under 5 MPa of syngas at 100 ℃. As demonstrated in Table 1, all the ligands exhibited excellent selectivity for aldehydes and regioselectivity for linear aldehyde (3-phenylbutanal, >97.0%). L1 and L3, which contained a di(N-pyrrolyl)phosphino group, resulted in much higher activity. In contrast, L2, bearing the N-carbazolyl group with a larger steric hindrance, resulted in relatively lower activity. L4- L6, which contained triphenylphosphine and its derivatives, also gave lower activity, and the one with an electron-withdrawing substituent -CF3 (L5) demonstrated higher activity compared to the one with the electron-donating substituent -OCH3 (L6) (Table 1, entries 4 and 5). Of the tested ligands, L1, which contained an electron-withdrawing N-pyrrolyl group and caused less steric hindrance, is the best ligand in terms of activity and regioselectivity. This is because the electron-withdrawing group substituted on the phosphorus weakens the Rh-carbonyl bond and thus favors the insertion of CO and the formation of Rh-acyl active species. In addition, a smaller ligand might somehow facilitate the coordination of α-methylstyrene to the Rh center [7], and thus afford 3-phenylbutanal as the dominant product. 1H NMR (400 MHz, CDCl3): δ 9.73 (s, 1H), 7.42-7.20 (m, 5H), 3.38 (s, 1H), 2.79 (d, 2H, J = 14.9 Hz), 1.34 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 201.90, 145.50, 128.72, 126.81, 51.76, 34.30, 22.22 (see Supporting information).
|
|
Table 1 Effect of ligand on Rh-catalyzed hydroformylation of α-methylstyrene.a |
The hydroformylation is highly dependent on the reaction conditions; therefore, the optimization of L1/Rh molar ratio, initial pressure, and reaction temperature was performed in the presence of Rh(acac)(CO)2/L1 as catalyst, and the results were shown in Table 2. When L1/Rh molar ratio of 5:10 was used, high activity (Table 2, entries 1 and 2) could be achieved at 100 ℃. Further increasing the L1/Rh molar ratio decreases the activity (Table 2, entry 3). Considering the stability of the catalyst, a L1/Rh molar ratio of 10 is selected for the sequent experiments. A clear impact of the reaction temperature on the activity was observed (Table 2, entries 4-8). For instance, the reaction rate was unsatisfactory at 90 ℃, while at 110 ℃ a high activity was achieved. From entries 4 to 8, it is not difficult to find that the chemoselectivity of the aldehyde decreased slightly when the temperature increased, because high temperatures were likely to benefit the hydrogenation of α-methylstyrene, which was confirmed by the observation of 2-phenylpropane via GC. The total pressure of syngas (CO/H2: 1/1) was also a key element in the hydroformylation. Increasing the pressure from 4 to 6 MPa resulted in higher activity and selectivity for aldehydes. The catalytic system has good activity: when the S/C was increased from 1000 to 3067, TOF of up to 2496 h-1 was attained and the conversion of a-methylstyrene was maintained at 79.4%-81.4% (Table 3, entries 1-3). The highest TOF (5786 h-1) was achieved at S/C of 10333 (Table 3, entry 6), although the conversion decreased to 56.0%. To our knowledge, this is the best result for the hydroformylation of a-methylstyrene so far [1, 2].
|
|
Table 2 Effect of reaction conditions on Rh-catalyzed hydroformylation of α-methylstyrene.a |
|
|
Table 3 Effect of substrate/catalyst molar ratio on Rh-catalyzed α-methylstyrene hydroformylation.a |
The process of styrene hydroformylation may form stable η3-allyl-Rh complexes, which could contribute to the formation of branched aldehyde [8]. In the case of α-methylstyrene, the steric hindrance caused by the phenyl and methyl groups on C-1 predisposes it to the formation of η1-allyl-Rh complexes. This ensured the linear aldehyde 3-phenylbutanal as the dominant product (> 99.0%).
4. ConclusionIn summary, the influence of the steric and electronic properties of the ligand on the activity and the regioselectivity was investigated in the Rh-catalyzed hydroformylation of α-methylstyrene. The results demonstrated that tris(N-pyrrolyl)phosphine L1 with good π-acceptability could efficiently improve the catalytic activity, and the steric hindrance of a-methylstyrene at α-C effectively enhanced the regioselectivity toward the linear aldehyde. A high TOF (5786 h-1) with 99% yield of linear aldehyde was obtained at relatively mild conditions (syngas pressures 6 MPa, 110 ℃), when Rh(acac)(CO)2/L1 was used as the catalyst, which is the highest activity reported for the hydroformylation of α-methylstyrene so far.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.01.028.
| [1] | A.C.J. Koeken, N.M.B. Smeets. A bulky phosphite modified rhodium catalyst for efficient hydroformylation of disubstituted alkenes and macromonomers in supercritical carbon dioxide. Catal. Sci. Tech. 3 (2013) 1036–1045 |
| [2] | T.J. Kwok, D.J. Wink. Characterization and application of catalytic regioselective hydroformylation with a cationic bis(dioxaphospholane)rhodium catalyst precursor. Organometallics 12 (1993) 1954–1959 |
| [3] | R. Jackstell, H. Klein, M. Beller, K.D. Wiese, D. Röttger. Synthesis of pyrrolyl-, indolyl-, and carbazolylphosphanes and their catalytic application as ligands in the hydroformylation of 2-pentene. Eur. J. Org. Chem (2001) 3871–3877 |
| [4] | C.Y. Zheng, M. Mo, H.R. Liang, et al. Rhodium/bisphosphite catalytic system for hydroformylation of styrene and its derivatives. Appl. Organomet. Chem. 27 (2013) 474–478 |
| [5] | P. Suomalainen, H.K. Reinius, H. Riihimaki, et al. Hydroformylation of 1-hexene and propene with in situ formed rhodium phosphine catalysts. J. Mol. Catal. A Chem. 169 (2001) 67–78 |
| [6] | H. Gulyas, A. Szollosy, P. Szabo, P. Halmos, J. Bakos. Preparation of new sulfonated triarylphosphanes:control of the selectivity by structural assistance. Eur. J. Org. Chem (2003) 2775–2781 |
| [7] | Y.G. Yan, X.W. Zhang, X.M. Zhang. A tetraphosphorus ligand for highly regioselective isomerization-hydroformylation of internal olefins. J. Am. Chem. Soc. 128 (2006) 16058–16061 |
| [8] | E. Boymans, M. Janssen, C. Muller, M. Lutz, D. Vogt. Rh-catalyzed linear hydroformylation of styrene. Dalton Trans. 42 (2012) 137–142 |
 2016, Vol. 27
2016, Vol. 27