b Institute of New Catalytic Materials Science, Key Laboratory of Advanced Energy Materials Chemistry Ministry of Education, College of Chemistry, Nankai University, Tianjin 300071, China ;
c Jiangsu Province Key Laboratory of Fine Petrochemical Engineering, Changzhou University, Changzhou 213164, China
Organic molecules in organisms with specific functional groups have significant influence on the growth of inorganic crystals [1]. Proteins,through their unique and specific interactions with other macromolecules and inorganics,dominate structures and functions of all biological hard and soft tissues in organisms [2]. Amino acids also as one of the most important biomolecules in organisms have attracted great interest [3, 4, 5, 6, 7, 8, 9, 10, 11]. For example,Matsumoto et al. [4] showed glycine,etc. has great influence on the size,solubility and morphology of hydroxyapatite adsorbed amino acids. He et al. [8] synthesized shuttle-like scrolled nanotubes of tellurium and tellurium nanowires in the single presence of serine,lysine and histidine. Thus,amino acids can be used as an efficient crystal growth modifier to prepare specific structural materials.
Rare earth molybdates are very important due to their potential applications as catalysts [12, 13, 14, 15, 16],phosphors [17, 18, 19, 20, 21],thermal expansion materials [22, 23, 24],etc. Among them,cerium(Ⅲ) molybdate has attracted much attention. For example,Kuang et al. [12, 15] found Ce2(MoO4)3 nanoparticles show better selectivity to partial oxidation of toluene to benzaldehyde than the other molybdates. Yousefi et al. [16] studied the adsorption behavior of cerium(Ⅲ) molybdate nanoparticles. Kartsonakis et al. [25] prepared cerium molybdate nanocontainers which can be used to suppress the corrosion of aluminum alloys in a corrosive environment. However,there are still few reports about the control of the morphology of cerium(Ⅲ) molybdate,especially for tuning the topology of its assemblies. Considering the efficient role of amino acids in crystal growth,it came to our attention that it would be interesting to fabricate cerium molybdate microparticles assisted by amino acids.
Herein,we report a solution-based method assisted by amino acids to prepare cerium(Ⅲ) molybdate microspheres. We studied the role of lysine and other amino acids on the morphologic control of cerium(Ⅲ) molybdate crystals. In the presence of lysine,morphologic cerium(Ⅲ) molybdate microspheres of three-dimensional or two-dimensional centripetal assemblies are obtained. Moreover,amino acids with hydrophilic "R" groups tend to induce nucleation and result in spherical assemblies formed by nanoparticles or nanoflakes. While ones with weaker hydrophilic "R" groups tend to induce growth and form spherical assemblies by microflakes.
2. Experimental 2.1. The preparation of cerium molybdateAll chemicals are analytically pure and are used without further purification. For the preparation of cerium(Ⅲ) molybdate,0.28 g (NH4)6Mo7O24·4H2O and 0.37 g CeCl3·7H2O were each separately dissolved in 10 mL deionized water. Then amino acid was added to the CeCl3 solution under magnetic stirring. Then 10 mL CeCl3 and amino acids mixed solution were added to 10 mL ammonium molybdate solution in strongly stirring. Upon mixing the two solutions (resulting in a molar ratio of Ce3+/Mo6+ = 2:3),a pale yellow precipitate was observed immediately. This precursor suspension was transferred into a 30 mL Teflon-lined stainless steel autoclave,which was subsequently sealed and maintained at 180 °C for 24 h. Then,the autoclave was cooled to room temperature naturally. The product was centrifuged and washed several times with deionized water,and dried at 80 °C. To study the role of lysine content on the product,zero mmol,0.75 mmol,1.00 mmol and 1.50 mmol of lysine were added to the four CeCl3 solutions. To further study the role of different amino acids on the resulting product,0.75 mmol glycine,serine and glutamine were added to three CeCl3 solutions and the solutions processed as above.
2.2. CharacterizationX-ray powder diffraction (XRD) patterns were used to analysis the phase composition and structure of the sample obtained on a Rigaku D/Max 2500 v/pc X-ray diffractometer with Cu Kα radiation (λ = 0.15418 nm). Scanning electron microscopy (SEM) images were recorded on a Shimadu SS-550 microscope. Transmission electronic microscopy (TEM) images and high resolution transmission electronic microscopy (HRTEM) images were obtained on a JEM-2100F instrument at an acceleration voltage of 200 kV. The X-ray photoelectron spectroscopy (XPS) analysis was performed on a Kratos Axis Ultra DLD X-ray photoelectron spectrometer using Mg Kα radiation as the excitation source. The binding energies obtained in the XPS analysis were corrected for specimen charging by referencing the C1s orbital to 284.60 eV. The ICP elemental and TGA analyses performed on Vista-MPX and TGA/DSC1 were necessary to discuss the composition of samples.
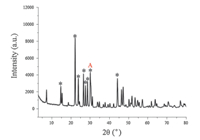
3. Results and discussion 3.1. The role of lysine contentThe typical XRD patterns of sample are showed in Fig. 1 (in the presence of 0.75 mmol lysine),the main XRD peaks are denoted by the symbol (*) and their 2θ values are list in Table S1 in Supporting information. For all prepared samples whether in the presence of lysine or not their XRD patterns (Figs. S1-S4 in Supporting information) are similar,which indicates lysine did not influence the composite of the samples. Their 2θ values of the main XRD peaks can be found in Tables S1-S4 in Supporting information. The interplanar spacing of diffraction peaks marked as 'A' in Fig. 1 is 0.29 nm. Diffraction peaks in Fig. 1 is very sharp,so it can be inferred that the prepared sample is crystalline as well. Unfortunately,we failed to match the measured XRD patterns with the standard XRD patterns of cerium molybdate crystals. To confirm the composition of samples,we utilized XPS,ICP elemental and TGA analysis.
|
Download:
|
| Fig. 1. The typical XRD patterns of sample. | |
The SEM images of the prepared samples in the presence of different levels of lysine content were obtained. Without lysine,the spherical products (Fig. 2a1 and a2) are assembled by nanoparticles and microflakes. In the presence of lysine,the cerium(Ⅲ) molybdate microspheres (Fig. 2b1-d2) are assembled by microflakes. The microsphere sizes vary but the majority of diameters are ~100 μm. When adding 0.75 mmol lysine,the morphology of the sample is effectively controlled. The threedimensional centripetal cerium(Ⅲ) molybdate microspheres are assembled by microflakes. The microflakes are 200-400 nm in thickness,5-10 μm in width and ~20 μm in length. When adding 1.00 mmol lysine,the three-dimensional centripetal cerium(Ⅲ) molybdate microspheres are assembled by microflakes and the diameter is ~50 μm. The microflakes are ~100 nm in thickness and the edge is irregular. When adding 1.50 mmol lysine,the morphology of the sample is also centripetal microspheres and the diameter is ~50 μm. The microflakes are ~100 nm in thickness. While the self-assembly of microflakes changes from 3D topology into 2D topology. As a consequence of the above,with the increase of lysine,the microflakes are thinner in thickness. When the lysine content reaches a certain level,the thickness of the microflakes remains unchanged. Meanwhile,the diameter of microspheres become smaller and the self-assembly of microflakes gradually change from three-dimensional into twodimensional topology. Whether adding amino acids or not,spherical products were obtained. While in the presence of lysine,the centripetal assembled products are obtained. It indicates that lysine cannot promote products forming into spheres but promote products forming centripetal assembled morphology.

|
Download:
|
| Fig. 2. The SEM images of samples in the presence lysine at 0 mmol (a1 and a2), 0.75 mmol (b1 and b2), 1.00 mmol (c1 and c2), and 1.50 mmol (d1 and d2); and the TEM image (e), HRTEM image (f) and related Fourier transform (g) of samples in the presence of 1.50 mmol lysine. | |
We also performed transmission electron microscopy (TEM) analyses and high resolution transmission electronic microscopy (HRTEM) on samples in the presence of 1.50 mmol lysine. As shown in Fig. 2e,the TEM image indicates the prepared sample is flake in morphology. According to the HRTEM image (Fig. 2f),the synthesized sample is crystalline as well and the interplanar spacing is 0.29 nm. By calculation,0.29 nm is consistent with the interplanar spacing of the diffraction peak,2θ = 30.80° (marked as 'A' in Fig. 1). Further,we performed a Fourier transform to HRTEM image. As shown in Fig. 2g,the result shows that the selected area is hexagonal crystal.
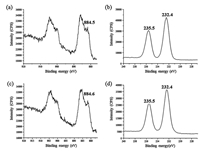
3.2. XPS analysisTo confirm the compositions of the samples,the X-ray photoelectron spectroscopic (XPS) analysis was performed. The results are shown in Fig. 3. In the absence of an amino acid,the binding energies of Ce 3d5/2 is 884.5 eV (Fig. 3a),which is attributed to Ce3+ and the binding energy of Mo 3d5/2 and Mo 3d3/2 are 232.5 eV and 235.7 eV (Fig. 3b),respectively,which is attributed to Mo6+ [26]. By calculation,the molar ratio of Ce3+/ Mo6+ is 2:3. It is inferred that the molecular formula of the crystals might be Ce2(MoO4)3·nH2O. The mismatching with the standard XRD pattern can be ascribed to different amounts of crystal water in the prepared cerium molybdate. In the presence of lysine,the XPS spectra of the sample are the same as the sample obtained without amino acids. That is to say,lysine might have no obvious influence on the composition of the example.
|
Download:
|
| Fig. 3. XPS spectra of samples in the absence of amino acid: (a) Ce3d, (b) Mo3d and in presence of 0.75 mmol lysine: (c) Ce3d, (d) Mo3d. | |
We further performed the ICP elemental and TGA analyses (Table S9 in Supporting information) to study the composition of prepared cerium molydate. From the ICP elemental analysis,the atomic ratio of Ce/Mo of the two typical samples are 2:3.49 (using 0.75 mmol glutamine) and 2:3.28 (using 1.5 mmol lysine),respectively. Thus,the atomic ratio of Ce/Mo of the samples is close to 2:3,which is consistent with the results of the XPS spectra (Fig. 3). According to the TGA analysis,within 200°C,the crystal water content of samples obtained without amino acid and using 1.5 mmol lysine is 6.73% and 0.81%,respectively. Correspondingly,their formulas are estimated to be Ce2(MoO4)3·3.0 H2O and Ce2(MoO4)3·0.34 H2O,respectively.
The adsorption behavior of cerium(Ⅲ) molybdate nanoparticles [16] has also been reported. Since the toxicity of cerium(Ⅲ) molybdate is environmentally low,the adsorption ability of the cerium molybdate is evaluated here using 10 ppm organic dyes (methylene blue,hereafter MB) as the target molecules (Fig. S8 in Supporting information). Results show that within 20 min,the adsorption for MB by the prepared samples reach 78.90% (obtained using 0.75 mmol lysine),76.33% (using 1.0 mmol lysine) and 48.28% (using 1.5 mmol lysine),respectively. Among them,the cerium molybdate obtained using 0.75 or 1.0 mmol lysine show the best adsorption ability of MB. The discrepancy observed in the adsorption capacity may be caused by the morphology. The samples obtained using 0.75 and 1.0 mmol lysine are 3D topography,while the sample obtained with 1.5 mmol lysine is 2D topography. The specific surface area of the former is larger than the latter and thus,exhibits a larger adsorption capacity.
3.3. The role of other amino acidsThe XRD patterns of all prepared samples in the presence of 0.75 mmol glycine (Fig. S5 in Supporting information),serine (Fig. S6 in Supporting information) and glutamine (Fig. S7) are similar,indicating the presence of amino acids cannot influence the composition of the samples. Their 2θ values of the main XRD peaks can be found in Tables S5-S7 in Supporting information. The SEM images for all the samples in the presence of different amino acids were taken. As shown in Fig. 4,in the presence of glycine,the morphology of the sample is three-dimensional microsphere having diameters of 70-150 μm and assembled by microflakes. The microflakes are 200-400 nm in thickness. In the presence of serine,morphology of the sample is microsphere assembled by tiny cerium(Ⅲ) molybdate nanoflakes with diameters of 30- 80 μm. The self-assembly of products has no definite centripetal. In the presence of glutamine,the morphology of the product has a diameter of 100 μm and is microspherical assembled by tiny cerium(Ⅲ) molybdate nanoparticles. Combining the XRD patterns and SEM images,we can infer that amino acids cannot influence the composite of the samples,but have an influence on the morphology of prepared samples.

|
Download:
|
| Fig. 4. SEM images of samples in the presence of 0.75 mmol glycine (a1 and a2), serine (b1 and b2), glutamine (c1 and c2). | |
From the structural formula of amino acids (Table S9 in Supporting information),the R group of glycine,lysine,serine,glutamine is -H,-(CH2)4NH2,-CH2OH,-(CH2)2CONH2,respectively. Moreover,the hydrophilic property of serine and glutamine is stronger than glycine and lysine. According to SEM images,serine and glutamine trend to induce nucleation and form microsphere assembled as nanoparticles and nanoflakes. While glycine and lysine trend to induce growth and form microsphere assembled as microflakes. This indicates that amino acids with hydrophilic "R" groups trend to induce nucleation and spherical assemblies assembled as either nanoparticles or nanoflakes; while those with weaker hydrophilic "R" groups trend to induce growth and yield spherical assemblies assembled by microflakes.
4. ConclusionIn our work,we found that lysine,glycine,serine and glutamine can effectively control the morphology of cerium(Ⅲ) molybdate. In the presence of lysine,we successfully prepared cerium(Ⅲ) molybdate with three-dimensional or two-dimensional centripetal assembled morphology. When adding 1.50 mmol lysine,two-dimensional centripetal assembled products were obtained. Thus,amino acid can effectively control the morphology of cerium(Ⅲ) molybdate and,moreover,the amino acids with strong hydrophilic "R" groups tend to induce nucleation and result in spherical assemblies formed by nanoparticles and nanoflakes. While those with weak hydrophilic "R" groups tend to induce growth and spherical assemblies assembled by microflakes.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.12.023.
| [1] | A. Berman, J. Hanson, L. Leiserowitz, et al. Biological control of crystal texture:a widespread strategy for adapting crystal properties to function. Science 259 (1993) 776–779 |
| [2] | M. Sarikaya, C. Tamerler, A.K.Y. Jen, et al. Molecular biomimetics:nanotechnology through biology. Nat. Mater. 2 (2003) 577–585 |
| [3] | C.A. Orme, A. Noy, A. Wierzbicki, et al. Formation of chiral morphologies through selective binding of amino acids to calcite surface steps. Nature 411 (2001) 775–779 |
| [4] | T. Matsumoto, M. Okazaki, M. Inoue, et al. Crystallinity and solubility characteristics of hydroxyapatite adsorbed amino acid. Biomaterials 23 (2002) 2241–2247 |
| [5] | H.G. Zhang, Q.S. Zhu, Y. Wang. Morphologically controlled synthesis of hydroxyapatite with partial substitution of fluorine. Chem. Mater. 17 (2005) 5824–5830 |
| [6] | H. Tong, W.T. Ma, L.L. Wang, et al. Control over the crystal phase, shape, size and aggregation of calcium carbonate via a L-aspartic acid inducing process. Biomaterials 25 (2004) 3923–3929 |
| [7] | A.H. Lu, B. Spliethoff, F. Schueth. Aqueous synthesis of ordered mesoporous carbon via self-assembly catalyzed by amino acid. Chem. Mater. 20 (2008) 5314–5319 |
| [8] | Z.B. He, S.H. Yu, J.P. Zhu. Amino acids controlled growth of shuttle-like scrolled tellurium nanotubes and nanowires with sharp tips. Chem. Mater. 17 (2005) 2785–2788 |
| [9] | P.V. Dhanaraj, G. Bhagavannarayana, N.P. Rajesh. Effect of amino acid additives on crystal growth parameters and properties of ammonium dihydrogen orthophosphate crystals. Mater. Chem. Phys. 112 (2008) 490–495 |
| [10] | Q.Y. Lu, F. Gao, S. Komarneni. Biomolecule-assisted reduction in the synthesis of single-crystalline tellurium nanowires. Adv. Mater. 16 (2004) 1629–1632 |
| [11] | T. Yokoi, J. Wakabayashi, Y. Otsuka, et al. Mechanism of formation of uniformsized silica nanospheres catalyzed by basic amino acids. Chem. Mater. 21 (2009) 3719–3729 |
| [12] | W.X. Kuang, Y.N. Fan, K.D. Chen, et al. Partial oxidation of toluene over ultrafine mixed Mo-based oxide particles. J. Catal. 186 (1999) 310–317 |
| [13] | W.X. Kuang, Y.N. Fan, L. Liu, et al. Study on the reactivity of ultrafine ceriummolybdenum oxide particles in the absence of molecular oxygen. Catal. Lett. 61 (1999) 173–178 |
| [14] | W.X. Kuang, Y.N. Fan, Y. Chen. Catalytic properties of ultrafinemolybdenum-cerium oxide particles prepared by the sol-gel method. Catal. Lett. 50 (1998) 31–35 |
| [15] | W.X. Kuang, Y.I. Fan, K.W. Yao, et al. Preparation and characterization of ultrafine rare earth molybdenum complex oxide particles. J. Solid State Chem. 140 (1998) 354–360 |
| [16] | T. Yousefi, A.R. Khanchi, S.J. Ahmadi, et al. Cerium(Ⅲ) molybdate nanoparticles:synthesis, characterization and radionuclides adsorption studies. J Hazard. Mater. 215 (2012) 266–271 |
| [17] | N. Zhang, W. Bu, Y. Xu, et al. Self-assembled flowerlike europium-doped lanthanide molybdate microarchitectures and their photoluminescence properties. J. Phys. Chem. C 111 (2007) 5014–5019 |
| [18] | S.F. Wang, K.K. Rao, Y.R. Wang, et al. Structural characterization and luminescent properties of a red phosphor series:Y2-xEux(MoO4)3(x=0.4-2.0). J. Am. Ceram. Soc. 92 (2009) 1732–1738 |
| [19] | Y. Tian, X. Qi, X. Wu, et al. Luminescent properties of Y2(MoO4)3:Eu3+ red phosphors with flowerlike shape prepared via coprecipitation method. J. Phys. Chem. C 113 (2009) 10767–10772 |
| [20] | L.K. Bharat, V.R. Bandi, J.S. Yu. Polyol mediated solvothermal synthesis and characterization of spindle shaped La2(MoO4)3:Eu3+ phosphors. Chem. Eng. J. 255 (2014) 205–213 |
| [21] | Y. Zhang, A. Zheng, X. Yang, et al. Controlled synthesis, characterization and photoluminescence property of olive-like tetragonal α-Nd2(MoO4)3. Mater Res. Bull. 47 (2012) 2364–2368 |
| [22] | S. Sumithra, A.M. Umarji. Negative thermal expansion in rare earth molybdates. Solid State Sci. 8 (2006) 1453–1458 |
| [23] | C.P. Romao, K.J. Miller, M.B. Johnson, et al. Thermal, vibrational, and thermoelastic properties of Y2Mo3O12 and their relations to negative thermal expansion. Phys. Rev. B:Condens. Matter 90 (2014) 024305–0243013 |
| [24] | J.S.O. Evans, T.A. Mary, A.W. Sleight. Negative thermal expansion materials. Phys. B:Condens. Matter 241-243 (1997) 311–316 |
| [25] | I.A. Kartsonakis, G. Kordas. Synthesis and characterization of cerium molybdate nanocontainers and their inhibitor complexes. J. Am. Ceram. Soc. 93 (2010) 65–73 |
| [26] | J.F. Moulder, W.F. Stickle, P.E. Sobol, et al., Chapter Ⅱ:standard ESCA spectra of the elements and line energy information, in:J. Chastain (Ed.), Handbook of X-ray Photoelectron Spectroscopy, Perkin-Elmer Corporation, Physical Electronic Division, USA, 1992, pp. 78-79. |
 2016, Vol. 27
2016, Vol. 27 


