Imidacloprid (E)-1-(6-chloro-3-pyridylmethyl)-N-nitroimidazolidin- 2-ylideneamine (Fig. 1) is one of the most used neonicotinoid for the crop protection worldwide due to its low soil persistence and high insecticidal activity at a very low application rate [1]. It acts as antagonist by binding to postsynaptic nicotinic receptors in the insect’s central nervous system resulting in the paralysis and death of insects [2]. IMD is the active substance of many commercial insecticides such as Confidor,Gaucho,Prestige,Admire,and Provado [3]. However,the intensive use of this insecticide in agriculture and the improper storage is a source of contamination of the environment. For these reasons,a range of methods have been utilized for the detection and quantification of IMD,such as high performance liquid chromatography [4, 5, 6, 7],spectrophotometry [8],spectrofluorimetry [9, 10],capillary electrophoresis [11],micellar electrokinetic chromatography [12] and enzyme-linked immunosorbent assay (ELISA) [13]. However,these methods suffer from some disadvantages such as high cost,long analysis time and requirement for sample pretreatment when some procedures as derivatization,extraction and purification are usually included.

|
Download:
|
| Fig. 1. Mesomeric form of imidacloprid. | |
Electroanalytical processes have been successfully applied for the determination of inorganic and organic compounds,including pesticides,in many environmental matrices,due to their good sensitivity,short time analysis,low-cost,and the possibility of sample analysis without extractions or pretreatments [14, 15, 16]. Several electrode materials such as glassy carbon [17],modified glassy carbon electrode [18, 19],carbon paste [20],modified carbon paste electrode [21, 22],carbon ceramic [23],bismuth modified electrode [24],modified silver electrode [25],and dropping mercury electrode [26, 27],were used as cathodes for the detection and quantification of this insecticide.
Differential pulse polarography and square-wave adsorptive stripping voltammetry were used for the determination of IMD at hanging mercury drop electrode [26, 27]. Two reduction peaks were observed in large pH ranges in both methods. The first peak corresponds to a four-electron transfer to give the hydroxylamine derivative and the second correspond to a two electron-transfer to give the corresponding amine (Scheme 1). The limits of detection (LOD) were 0.01 μmol L-1 and 0.02 μmol L-1,respectively.
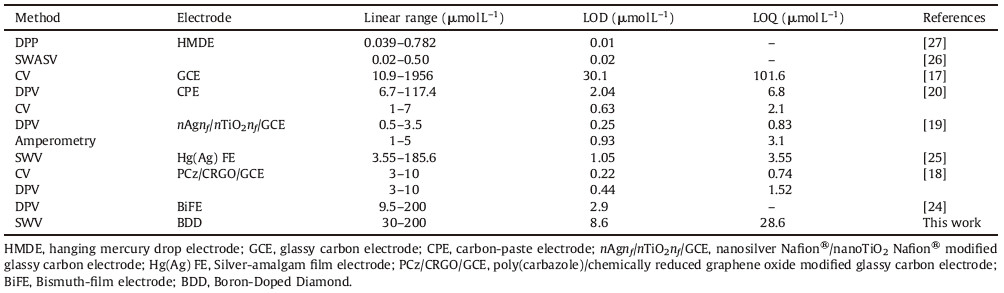
Voltammetric determination of IMD at glassy carbon electrode has been reported [17]. The reduction peak is observed at high scan rate of 500 mV s-1. The reported detection limit was 30.11 μmol L-1,which is too high for carrying out trace analysis. Differential pulse voltammetry (DPV) determination of IMD at carbon paste electrode has been reported [20] with the LOD as 2.04 μmol L-1. A copper(Ⅱ) phthalocyanine modified carbon ceramic electrode fabricated by a sol-gel method [23],and a nano silver-Nafion/TiO2-Nafion composite modified GCE [19],were used for the detection of IMD by DPV technique,with the LOD 0.28 μmol L-1 and 0.25 μmol L-1,respectively for both electrodes. Recently,Lei et al. [18] describe a method for the determination of IMD on a GCE modified with poly(carbazole)/chemically reduced graphene oxide. This modified electrode was used as a sensing electrode for the detection of IMD. CV and DPV were used as sensing techniques. The LOD and LOQ values of CV are 0.22 μmol L-1 and 0.74 μmol L-1,respectively,while those values of DPV are 0.44 μmol L-1 and 1.52 μmol L-1,respectively. The LOD and LOQ for the reduction of IMD on various electrodes are presented in Table 1.
|
|
Table 1 Comparison of analytical parameters for the determination of IMD on various electrodes. |
None of the previously conducted research works reports the electrochemical determination of the IMD on boron-doped diamond (BDD) electrode. The development of a novel electrochemical technique using BDD electrode can furnish the priceless services in the monitoring of the compounds that are important in terms of human health defense,environment and food chemistry. BDD electrodes have been excellently used as an alternative to other conventional electrodes (e.g.,glassy-carbon or platinum electrodes) and are particularly attractive in electroanalytical applications for pesticides [28, 29, 30, 31, 32, 33, 34, 35]. It has several important characteristics such as an inert surface with low adsorption proprieties,remarkable corrosion stability,even in strong acidic media,an extremely wide potential window in aqueous solutions (up to 3.5 V) and high reproducibility of electrochemical responses [36, 37, 38, 39].
The aim of this work is to develop a simple,fast and sufficiently sensitive electroanalytical methodology for the detection and quantification of IMD in the commercial formulation (Confidor 200 SL) using square-wave voltammetry at a BDD electrode. The results of the developed electroanalytical method were compared with the UV spectrophotometric and HPLC methods.
2. ExperimentalReagents: All solutions containing different amounts of IMD taken from an emulsifiable concentrate (Confidor 200 SL Bayer Crop Science) containing 200 g L-1 IMD were prepared with ultrapure water. These solutions were kept in the dark at 4 °C. The supporting electrolyte was sodium sulfate (Na2SO4) 0.05 mol L-1 provided by Biochem. Sulfuric acid (H2SO4) and sodium hydroxide (NaOH) were supplied by Merck and added to the required pH values covering the pH range of 3.0-11.0. Before each experiment,the solution was purged with nitrogen for 5 min to remove oxygen and kept away from any agitation to achieve a balance between the electrodes and the solution.
Apparatus: Square-wave voltammetry measurements were performed using a potentiostat-galvanostat (VoltaLab PST050),and a conventional three-electrode cell at 25 °C. BDD was used as working electrode for voltammetric experiments. A platinum wire was used as a counter electrode and a saturated calomel electrode (SCE) was used as reference electrode. The BDD electrode (0.07 cm2) was provided by the Swiss Center for Electronics and Microtechnology (CSEM). It was synthesized by the hot filament chemical vapor deposition technique (HF-CVD) on single-crystal p-type Si h1 0 0i wafers (1-3 mΩ cm,Siltronix) [40]. The doping level of boron in the diamond layer,expressed as B/C ratio,was about 3500 ppm. In order to obtain an active surface and reproducible electrochemical response,the BDD electrode was subjected to potential cycling conditions in 0.05 mol L-1 sodium sulfate between -3.0 V and +3.0 V at a high scan rate of 5.0 V s-1 for 120 s. The electrode surface state was controlled by obtaining the same shape of the voltammogram recorded between -0.4 and -1.6 V at a scan rate of 100 mV s-1.
UV spectrophotometric instrument: The spectrophotometric analysis was performed with UV-vis spectro-photometer (Shimadzu 1650 PC) with a Silicon photodiode detector for the measurement of the complete ultra-violet to visible light spectrum. The UV-vis spectra were recorded in a quartz cuvette of 1 cm path length. The spectrophotometer was connected to a computer have the software UV-Prob version 2.21 that was used for all the spectrophotometric measurements and data processing. The solutions were scanned from 200 nm to 400 nm. All measurements were performed at room temperature.
High performance liquid chromatography instrument: HPLC measurements (Fa. Knauer,Berlin,Germany) were carried out with HP 1081 series HPLC system with UV detection. A reverse phase C18 (cartridge) column was used (150 mm length,46 mm ID). The mobile phase was a mixture of acetonitrile and ultrapure water in ratio 8:2,v/v. The separation was performed in the isocratic regime and the flow rate was 1 mL min-1. IMD was detected at a wavelength of 270 nm with the retention time of 2.5 min. For the external calibration,at least three replications were performed for each standard concentration. The mean value was then used for calibration curve.
Preparation of plum red juice: Mature plum fruit was purchased from the local market of Sfax-Tunisia. Each fruit sample was washed in ultrapure water and processed mechanically by braun MP-80 multipress juice extractor. The suspension was filtered through a 0.45 μm milli-pore filter (Watman filter paper),centrifuged for 20 min until a clear sample,then,the obtained juice was fortified by the IMD. The amount of IMD in the plum juice was evaluated by the standard addition method. The IMD content in the fruit juice was determined from the calibration curve of IMD concentration vs. peak current. The pH of the juice extracted was modified to 7.0 with appropriate volumes of NaOH solution. Finally,20 mL of the filtrate juice was added to the supporting electrolyte solution (0.05 mol L-1) in the voltammetric cell for measurement. All tests cited in this work were performed at 25 °C.
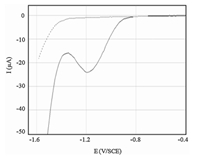
3. Results and discussionSWV technique was employed to understand the electrochemical process of IMD occurring at BDD electrode. Fig. 2 shows the square-wave voltammogram recorded in the absence and presence of 200 μmol L-1 IMD in a solution of 0.05 mol L-1 Na2SO4 at the BDD electrode. It is evident that,during the cathodic scan,a single reduction peak was observed at a potential of -1.21 V vs. SCE.
|
Download:
|
| Fig. 2. Square wave voltammograms of the BDD electrode immersed in 0.05 mol L-1 Na2SO4 solution after bubbling with nitrogen for 5 min: without (dashed line) and with (solid line) 200 μmol L-1 IMD. f = 100 Hz, △Ea = 50 mV, △Ei = 10 mV, pH 7.0. | |
The optimization of the analytical method involved a systematic study of the experimental parameters that affect the SWV response,namely,the pH of the medium,the frequency (f),the pulse amplitude (△Ea) and the scan increment (△Ei). The results of these studies will be separately presented in the sequence.
3.1. Effect of pHThe pH of the supporting electrolyte has a significant influence on the determination of IMD by affecting the peak current and peak potential. The effect of the pH value on the determination of IMD in the Na2SO4 solution at BDD electrode was carefully investigated in a wide pH range of 3.0 to 11.0. Fig. 3 illustrates the dependency of the IMD cathodic peak current and potential with pH. It could be seen that the cathodic peak current of IMD increases with the increase of pH value until it reaches 7.0 at higher pH values,the electrode response becomes not stable and the results are not reproducible,especially above pH 9.0. The best sensitivity and shape of the voltammograms were observed at pH 7.0. Therefore,pH 7.0 was chosen for the entire experiment. In addition,the peak potential Ep is shifted to more negative values with the increase of the pH value.

|
Download:
|
| Fig. 3. Dependence of peak current and peak potential on pH for 200 μmol L-1 IMD in 0.05 mol L-1 Na2SO4 solution at different pH (3.0-11.0). f = 100 Hz, △Ea = 50 mV, △Ei = 10 mV. | |
3.2. Effect of square-wave frequency
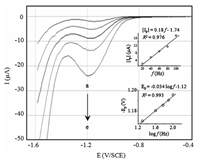
This parameter is important in SWV because it determines the intensity of the signal,which in turn,the sensitivity of the analytical method. Fig. 4 shows the square-wave voltammograms obtained for different frequency values in the range of 20 Hz to 100 Hz. The cathodic peak current increases with the increase of the frequency value. A linear relationship was obtained between the peak current (Ip) and the frequency (f) (inset of Fig. 4) according to the accepted theory of SWV [41]. This behavior corresponds to a totally irreversible system controlled by the adsorption of species on the electrode surface.
|
Download:
|
| Fig. 4. Square wave voltammograms of the BDD electrode immersed in a solution containing 0.05 mol L-1 Na2SO4 + 200 μmol L-1 IMD recorded at different frequency values: (a) 20; (b) 40; (c) 60; (d) 80; (e) 100 Hz. △Ea = 50 mV, △Ei = 10 mV, pH 7.0. | |
Moreover,the inset of Fig. 4 shows the dependence of Ep with log f,the slope of this straight line was used to calculate αn value according to the following equation:
| $\frac{{\Delta {E_p}}}{{\Delta \log f}} = - \frac{{2.3RT}}{{\alpha nF}}$ | (1) |
where α is the charge transfer coefficient,n the number of electrons involved in the reaction.
αn is equal to 1.73. From the literature [23],the number of electrons exchanged by the IMD is assumed to be 4 leading to the formation of hydroxylamine derivative. Therefore,the charge transfer coefficient α is about 0.43.
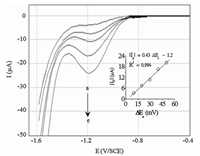
3.3. Effect of pulse amplitudeAnother important parameter involved in SWV is the pulse amplitude (△Ea). The effect of square-wave pulse amplitude on the reduction peak current of 200 μmol L-1 IMD in 0.05 mol L-1 Na2SO4 was studied from 10 mVto 50 mVat a frequency of 100 Hz. The results have indicated that the peak current increased from 3.33 μA to 20.3 μA when △Ea increased from 10 mV to 50 mV (Fig. 5). Therefore,at the pulse amplitude of 50 mV,the peak current which was found to be much sharper than the others. On the other hand,the inset of Fig. 5 allows to calculate the surface coverage (Γ) by the adsorbed IMD molecules according to the following equation [42]:
| $\frac{{\partial {I_p}}}{{\partial \Delta {E_a}}} = 500A{\alpha ^2}Ff\Delta {E_i}\Gamma $ | (2) |
|
Download:
|
| Fig. 5. Square wave voltammograms of the BDD electrode immersed in a solution containing 0.05 mol L-1 Na2SO4 + 200 μmol L-1 IMD recorded at different values of △Ea: (a) 10; (b) 20; (c) 30; (d) 40; (e) 50 mV. Inset: linear dependence of the peak current with △Ea. f = 100 Hz, △Ei = 10 mV, pH 7.0. | |
where ∂Ip/∂△Ea is the slope of the graph Ip vs. △Ea,A is the electrode area and △Ei is the scan increment. Thus,the quantity of IMD adsorbed on the electrode surface was estimated to be 6.88×10-10 mol cm-2.
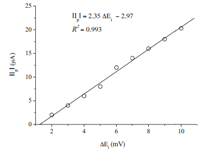
3.4. Effect of scan incrementThis parameter which allows the determination of the amount of potential changes between two data points in the experiment was investigated. The effect of the scan increment (△Ej) on the electrochemical reduction of 200 μmol L-1 IMD by SWV was studied in the range of 2 mV to 10 mV on the BDD electrode. Fig. 6 shows that the peak current enhanced with △Ei. Therefore,a scan increment of 10 mV was selected in the present study.
|
Download:
|
| Fig. 6. Linear dependence of the peak current (Ip) with scan increment (2-10 mV). f = 100 Hz, △Ea = 50 mV, pH 7.0. | |
3.5. Analytical determination of IMD
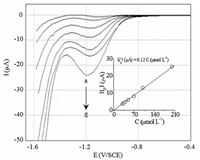
The validation of an analytical method aims at demonstrating that the analytical procedure is suitable for the intended use. It involves determination of accuracy,precision,repeatability,intermediate precision,reproducibility,specificity,limit of detection (LOD),limit of quantification (LOQ) and linearity range [43]. Fig. 7 shows the square-wave voltammograms obtained for the reduction of IMD at different concentrations (30-200 μmol L-1) under optimum experimental parameters. The inset in Fig. 7 shows the linear dependence of |Ip| vs. IMD concentration. The LOD and LOQ were calculated using the following equations [44].
| ${\rm{LOD = }}\frac{{3s}}{m}$ | (3) |
| ${\rm{LOQ = }}\frac{{10s}}{m}$ | (4) |
|
Download:
|
| Fig. 7. Square wave voltammograms of the BDD electrode immersed in a solution containing 0.05 mol L-1 Na2SO4 + IMD at different concentrations: (a) 30; (b); 40 (c) 50; (d) 60; (e) 70; (f) 100; (g) 200 μmol L-1. Inset: linear dependence of the peak current with the IMD concentrations. f = 100 Hz, △Ea = 50 mV, △Ei = 10 mV, pH 7.0. | |
where s is the standard deviation of the peak currents and m is the slope of the calibration curve.
The calculated values of LOD and LOQ were,respectively,8.60 μmol L-1 and 28.67 μmol L-1. Table 1 compares the performance of the proposed SWV approach with several electrochemical methods reported in the literature for IMD determination. Despite their general satisfactory sensitivities,several methods listed in Table 1 used modified electrodes,and complex biosensors whose preparation is laborious and expensive. It also requires skilled labor and involves several steps. However,the BDD electrode does not need everytime of pretreatment and it surface state remains stable after several tests. This electrode shows a better electrocatalytic activity towards the reduction of IMD. The repeatability and reproducibility of results are excellent. In fact,to evaluate these properties,two IMD solutions (50 μmol L-1 and 75 μmol L-1) were tested four times by SWV. The repeatability of the current measurement was 1.17% RSD for 50 μmol L-1 and 1.05% RSD for 75 μmol L-1. The reproducibility of the current measurement was also calculated 0.97% RSD for 50 μmol L-1 and 0.88% RSD for 75 μmol L-1. Low values of RSD showed the good precision of the proposed SWV method for the determination of IMD.
Various IMD concentrations were analyzed by HPLC in order to validate and compare the results obtained withSWV using the BDD electrode. The analytical curve in ultrapure water solutions was also obtained by the standard addition under the same interval for the SWV (30-200 μmol L-1). Fig. 8 shows the obtained chromatogram of IMD in aqueous solution containing 0.05 mol L-1 Na2SO4 at pH 7.0 and temperature 25 °C. The peak area increased with the increase in concentration. The inset in Fig. 8 corresponds to the analytical curve,i.e.,the linear dependence of the peak area with IMD concentration for the studied concentration interval. The calculated value of LOD was 7.61 μmol L-1 and the LOQ was 25.36 μmol L-1 (Table 2).

|
Download:
|
| Fig. 8. HPLC-UV chromatogram of 30 μmol L-1 IMD in 0.05 mol L-1 Na2SO4 solution at pH 7.0. Inset: linear dependence of the area with IMD concentration. Mobile phase: 80% acetonitrile, 20% ultrapure water (vol%), λ = 270 nm. | |
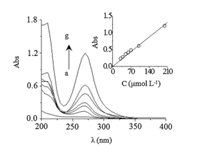
The UV spectrophotometry method was also developed with the objective to obtain comparative results for the validation of the electroanalytical method. The absorption spectra obtained for IMD exhibits one band at 270 nm (Fig. 9). The result shows a linear relationship between absorbance and IMD concentration in the same range of SWV. The LOD and LOQ were 8.42 μmol L-1 and 28.03 μmol L-1,respectively. The main results obtained with the three techniques are summarized in Table 2.
|
|
Table 2 Determination of IMD by SWV, UV spectrophotometry and HPLC. |
|
Download:
|
| Fig. 9. Evolution of the UV-vis spectra of IMD at different concentrations: (a) 30; (b) 40; (c) 50; (d) 60; (e) 70; (f) 100 and (g) 200 μmol L-1 in 0.05 mol L-1 Na2SO4 solution at pH 7.0. Inset: linear dependence of the absorbance with IMD concentration at λ = 270 nm. | |
3.6. Determining of IMD in plum fruit juice
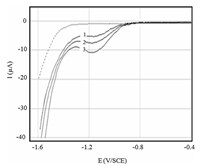
The possibility of applying the voltammetry technique to the determination of IMD in plum juice was also successfully tested by standard additions of IMD in the experimental section. Voltammograms of IMD in plum juice are given in Fig. 10. As can be seen from this figure,the juice of plum extracted from the unfortified plum sample by IMD (dashed line) did not block the BDD electrode surface and did not contain other voltammetrically active compounds,which are favorable facts for the IMD determination. All voltammograms recorded after addition of IMD show a single irreversible cathodic peak. The current of this peak allows the determination of IMD concentration using the calibration curve in Fig. 7. The result of this analysis is summarized in Table 3.
|
|
Table 3 Analytical parameters obtained at a BDD electrode for determination of IMD in Plum juice using SWV technique. |
|
Download:
|
| Fig. 10. Square wave voltammograms of the BDD electrode immersed in plum juice containing 0.05 mol L-1 + IMD. Plum juice: without (dashed line) and with (solid lines): successive standard additions of IMD. Concentration of IMD added: (1) 50, (2) 75, (3) 100 μmol L-1. f = 100 Hz, △Ea = 50 mV, △Ei = 10 mV, pH 7.0. | |
The recovery efficiencies (R %) for the different systems under investigation were calculated by using the following equation:
| $R\left( \% \right){\rm{ = }}\frac{{{{\left[{{\rm{IMD}}} \right]}_{{\rm{found}}}}}}{{{{\left[{{\rm{IMD}}} \right]}_{{\rm{added}}}}}} \times 100$ | (5) |
The precision and accuracy were tested with different standard solutions and the relative standard deviations (RSD) were calculated by using the following equation:
| ${\rm{RSD}}\left( \% \right){\rm{ = }}\frac{{{\rm{SD}}}}{x}$ | (6) |
where SD is the standard deviation of the mean current values obtained and x is the mean peak current value.
The analysis of the peak current showed that the obtained values of recovery ranged from 96.6% to 100.9% and the relative standard deviations (% RSD) ranged from 1.02% to 1.1%. In this study lower RDS % values were obtained. These results indicate that the BDD electrode retained its efficiency for the determination of IMD in real sample with satisfactory results.
4. ConclusionAccording to the literature,the electrochemical reduction of IMD on a BDD electrode was applied for the first time. SWV technique was developed to determination of this insecticide in commercial formulation (Confidor 200-SL). This BDD electrode showed good activity toward the electrochemical reduction of the IMD insecticide and high reproducibility results. A single and irreversible cathodic peak was occurred at a potential about -1.21 V vs. SCE in the presence of Na2SO4 as supporting electrolyte. The current of this peak was linearly dependent on the IMD concentration. After optimization of the experimental parameters the limit of detection LOD and the limit of quantification LOQ of IMD were found to be 8.6 μmol L-1 and 28.6 μmol L-1,respectively. A Good recoveries and low relative standard deviation reflect the high accuracy and precision of the proposed method. It can be concluded also that,the proposed voltammetry method can undoubtedly be considered as an effective to detect and quantify IMD in real samples such as,plum fruit.
| [1] | S.L. Chao, J.E. Casida. Interaction of imidacloprid metabolites and analogs with the nicotinic acetylcholine receptor of mouse brain in relation to toxicity. Pestic. Biochem. Physiol. 58 (1997) 77–88 |
| [2] | K. Matsuda, M. Shimomura, M. Ihara, M. Akamatsu, D.B. Sattelle. Neonicotinoids show selective and diverse actions on their nicotinic receptor targets:electrophysiology, molecular biology, and receptor modeling studies. Biosci. Biotechnol. Biochem. 69 (2005) 1442–1452 |
| [3] | C.D.S. Tomlin, The Pesticide Manual:A World Compendium, 12th ed., British Crop Protection Council, Farnham, United Kingdom, 2000, pp. 591-593. |
| [4] | M.L. Chin-Chen, J. Esteve-Romero, S. Carda-Broch. Determination of the insecticide imidacloprid in fruit juices using micellar high-performance liquid chromatography. J. AOAC Int. 92 (2009) 1551–1556 |
| [5] | K.C. Ting, E.G. Zhou, N. Saini. Determination of imidacloprid in fruits and vegetables by liquid chromatography with diode array and nitrogen-specific chemiluminescence detection. J. AOAC Int. 87 (2004) 997–1002 |
| [6] | H. Obana, M. Okihashi, K. Akutsu, Y. Kitagawa, S. Hori. Determination of acetamiprid, imidacloprid, and nitenpyram residues in vegetables and fruits by highperformance liquid chromatography with diode array detection. J. Agric. Food Chem. 50 (2002) 4464–4467 |
| [7] | S. Baskaran, R.S. Kookana, R. Naidu. Determination of the insecticide imidacloprid in water and soil using high-performance liquid chromatography. J. Chromatogr. A 787 (1997) 271–275 |
| [8] | A. Navalon, A. González-Casado, R. El-Khattabia, J.L. Vilchez, A.R. Fernández-Alba. Determination of imidacloprid in vegetable samples by gas chromatographymass spectrometry. Analyst 122 (1997) 579–581 |
| [9] | J.L. Vílchez, M.C. Valencia, A. Navalón, B. Molinero-Morales, L.F. Capitán-Vallvey. Flow injection analysis of the insecticide Imidacloprid in water samples with photochemically induced fluorescence detection. Anal. Chim. Acta 439 (2001) 299–305 |
| [10] | J.L. Vilchez, R. El-Khattabi, R. Blanc, A. Navalón. Photochemical-fluorimetric method for the determination of the insecticide imidacloprid in water samples. Anal. Chim. Acta 371 (1998) 247–253 |
| [11] | L. Sánchez-Hernández, D. Hernández-Domínguez, J. Bernal, et al. Capillary electrophoresis-mass spectrometry as a new approach to analyze neonicotinoid insecticides. J. Chromatogr. A 1359 (2014) 317–324 |
| [12] | A.S. Carretero, C. Cruces-Blanco, S.P. Durán, A.F. Gutié rrez. Determination of imidacloprid and its metabolite 6-chloronicotinic acid in greenhouse air by application of micellar electrokinetic capillary chromatography with solid-phase extraction. J. Chromatogr. A 1003 (2003) 189–195 |
| [13] | J.K. Lee, K.C. Ahn, O.S. Park, S.Y. Kang, B.D. Hammock. Development of an ELISA for the detection of the residues of the insecticide imidacloprid in agricultural and environmental samples. J. Agric. Food Chem. 49 (2001) 2159–2167 |
| [14] | T. Thriveni, J.R. Kumar, J.Y. Lee, N.Y. Sreedhar. Study of the voltammetric behaviour of the ethalfluralin and methalpropalin and its determination in environmental matrices at hanging mercury drop electrode. Environ. Monit. Assess. 151 (2009) 9–18 |
| [15] | H. Mercan, R. Inam. Determination of the fungicide anilazine in soil and river water by differential pulse polarography. Clean 36 (2008) 913–919 |
| [16] | R. İnam, E.Z. Gülerman, T. Sarigül. Determination of triflumizole by differential pulse polarography in formulation, soil and natural water samples. Anal. Chim. Acta 579 (2006) 117–123 |
| [17] | V.J. Guzsvány, F.F. Gaál, L.J. Bjelica, S.N. Okresz. Voltammetric determination of imidacloprid and thiamethoxam. J. Serb. Chem. Soc. 70 (2005) 735–743 |
| [18] | W. Lei, Q.J. Wu, W.M. Si, et al. Electrochemical determination of imidacloprid using poly(carbazole)/chemically reduced graphene oxide modified glassy carbon electrode. Sens. Actuators, B:Chem. 183 (2013) 102–109 |
| [19] | A. Kumaravel, M. Chandrasekaran. Electrochemical determination of imidacloprid using nanosilver Nafion®/nanoTiO2 Nafion® composite modified glassy carbon electrode. Sens. Actuators, B:Chem. 158 (2011) 319–326 |
| [20] | Z. Papp, I. Švancara, V. Guzsvány, K. Vytřas, F. Gaál. Voltammetric determination of Imidacloprid insecticide in selected samples using a carbon paste electrode. Microchim. Acta 166 (2009) 169–175 |
| [21] | Z. Papp, V. Guzsvány, I. Švancara, K. Vytřas. Voltammetric monitoring of photodegradation of Clothianidin, Nitenpyram and Imidacloprid insecticides using a tricresyl phosphate-based carbon paste electrode. Int. J. Electrochem. Sci. 6 (2011) 5161–5171 |
| [22] | V. Guzsvány, Z. Papp, J. Zbiljić, O. Vajdle, M. Rodić. Bismuth modified carbonbased electrodes for the determination of selected neonicotinoid insecticides. Molecules 16 (2011) 4451–4466 |
| [23] | M.R. Majidi, K. Asadpour-Zeynali, M. Bamorowat, M. Nazarpur. Determination of imidacloprid in tomato grown in Greenhouse based on copper (Ⅱ) phthalocyanine modified carbon ceramic electrode by differential pulse voltammetry. J. Chin. Chem. Soc. 58 (2011) 207–214 |
| [24] | V. Guzsvány, M. Kádár, Z. Papp, L. Bjelica, F. Gaál, K. Tóth. Monitoring of photocatalytic degradation of selected neonicotinoid insecticides by cathodic voltammetry with a bismuth film electrode. Electroanalysis 20 (2008) 291–300 |
| [25] | V. Guzsvány, J. Petrović, J. Krstić, et al. Renewable silver-amalgam film electrode for voltammetric monitoring of solar photodegradation of imidacloprid in the presence of Fe/TiO2 and TiO2 catalysts. J. Electroanal. Chem. 699 (2013) 33–39 |
| [26] | A. Guiberteau, T. Galeano, N. Mora, P. Parrilla, F. Salinas. Study and determination of the pesticide imidacloprid by square wave adsorptive stripping voltammetry. Talanta 53 (2001) 943–949 |
| [27] | A. Navalón, R. El-Khattabi, A. González-Casado, J.L. Vilchez. Differential-pulse polarographic determination of the insecticide imidacloprid in commercial formulations. Microchim. Acta 130 (1999) 261–265 |
| [28] | W. Dai, M.J. Li, H.J. Li, B.H. Yang. Amperometric biosensor based on nanoporous nickel/boron-doped diamond film for electroanalysis of L-alanine. Sens. Actuators, B:Chem. 201 (2014) 31–36 |
| [29] | L. Bandžuchová, L. Švorc, J. Sochr, J. Svítková, J. Chýlková. Voltammetric method for sensitive determination of herbicide picloram in environmental and biological samples using boron-doped diamond film electrode. Electrochim. Acta 111 (2013) 242–249 |
| [30] | L. Bandžuchová, L. Švorc, M. Vojs, et al. Self-assembled sensor based on borondoped diamond and its application in voltammetric analysis of picloram. Int. J. Environ. Anal. Chem. 94 (2014) 943–953 |
| [31] | A. Levent, Y. Yardım, Z. Şentürk. Electrochemical performance of boron-doped diamond electrode in surfactant-containing media for ambroxol determination. Sens. Actuators, B:Chem. 203 (2014) 517–526 |
| [32] | L. Švorc, M. Rievaj, D. Bustin. Green electrochemical sensor for environmental monitoring of pesticides:determination of atrazine in river waters using a borondoped diamond electrode. Sens. Actuators, B:Chem. 181 (2013) 294–300 |
| [33] | T.N. Rao, B.H. Loo, B.V. Sarada, C. Terashima, A. Fujishima. Electrochemical detection of Carbamate pesticides at conductive diamond electrodes. Anal. Chem. 74 (2002) 1578–1583 |
| [34] | K. Pecková, J. Musilová, J. Barek. Boron-doped diamond film electrodes-new tool for voltammetric determination of organic substances. Crit. Rev. Anal. Chem. 39 (2009) 148–172 |
| [35] | K. Peckova, J. Barek. Boron doped diamond microelectrodes and microelectrode arrays in organic electrochemistry. Curr. Org. Chem. 15 (2011) 3014–3028 |
| [36] | M. Hupert, A. Muck, R. Wang, et al. Conductive diamond thin-films in electrochemistry. Diamond Relat. Mater. 12 (2003) 1940–1949 |
| [37] | R.A. Medeiros, B.C. Lourencao, R.C. Rocha-Filho, O. Fatibello-Filho. Simultaneous voltammetric determination of synthetic colorants in food using a cathodically pretreated boron-doped diamond electrode. Talanta 97 (2012) 291–297 |
| [38] | G.R. Salazar-Banda, L.S. Andrade, P.A.P. Nascente, et al. On the changing electrochemical behaviour of boron-doped diamond surfaces with time after cathodic pre-treatments. Electrochim. Acta 51 (2006) 4612–4619 |
| [39] | H.B. Suffredini, V.A. Pedrosa, L. Codognoto, et al. Enhanced electrochemical response of boron-doped diamond electrodes brought on by a cathodic surface pre-treatment. Electrochim. Acta 49 (2004) 4021–4026 |
| [40] | A. Perret, W. Haenni, N. Skinner, et al. Electrochemical behavior of synthetic diamond thin film electrodes. Diamond Relat. Mater. 8 (1999) 820–823 |
| [41] | J.J. O'Dea, A. Ribes, J.G. Osteryoung. Square-wave voltammetry applied to the totally irreversible reduction of adsorbate. J. Electroanal. Chem. 345 (1993) 287–301 |
| [42] | M. Lovrić, Š. Komorsky-Lovrić, R.W. Murray. Adsorption effects in square-wave voltammetry of totally irreversible redox reactions. Electrochim. Acta 33 (1988) 739–744 |
| [43] | A.E. Bretnall, G.S. Clarke, Validation of analytical test methods, in:A. Satinder, S. Stephen (Eds.), Separation Science and Technology, Academic Press, New York, 2011, p.429. |
| [44] | D.A. Skoog, F.J. Holler, T.A. Nieman, Principles of Instrumental Analysis, fifth ed., Saunders College Publishing, Philadelphia, PA, 1998(pp. 13, 15-18). |
 2016, Vol. 27
2016, Vol. 27