b Department of Environmental Science and Engineering, Shandong University, Jinan 250199, China
The strict regulations of fuel specifications recently implemented in many countries for environmental protection have promoted the research demand of optimizing deep desulfurization technologies. Oil desulfurization technology can be simply divided into hydrodesulfurization (HDS) and non-hydrodesulfurization(NHDS).HDSis highly efficient in removing thiols,sulfides and disulfides,however,its investment and operation costs are both very high.Moreover,it is less successful for removing refractory sulfur species,such as dibenzothiophene (DBT) and its derivatives [1]. Oxidative desulfurization process (ODS) applied as one kind of NHDS methods,has shown to be one of themost promising techniques as it avoids use of hydrogen and can be conducted under mild condition. Different oxidants,such as hydrogen peroxide [2],molecular oxygen [3],and ozone [4],were used in the process to reduce sulfur in fuel.
The polyoxometalates (POMs) having strong Brønsted acidity enable them to be utilized as solid-acid catalysts in chemical reactions. The most obvious drawback is the relatively poor surface area for heterogeneous process,which confined the application of POMs as solid catalysts. Thus materials with high surface area are utilized to act as supports to improve the dispersion of POMs. Metal organic frameworks (MOFs) have emerged as a particular class of functional solid-state materials,owing to their large surface area,adjustable chemical composition and tunable pore structure. Recently,investigations relevant to applications ofMOFs in gas storage,separation,catalysis and drug delivery have been widely reported [5, 6, 7, 8]. Consequently,MOFs have been found to be suitable supports capable of incorporating POMs to form efficient heterogenous catalysts. However,at present,only a few examples can be found in literatures involving the use of POM@MOFs as catalysts for oxidative desulfurization,and the oxidant used was H2O2 [9, 10]. Taking environmental and economic concerns into account,the utilization of O2 as the oxidant for ODS ismost desirable,especially the advantages of air has received a universal attention. At present,O2 has been used for ODS with catalysts like metal oxides and salts [11, 12, 13],but these methods can only achieve low desulfurization efficiency. Murata reported a highly efficient oxidation of sulfur compoundswith O2 by using cobalt salts as catalysts and aldehydes as sacrificial materials,respectively [14]. However,use of sacrificial materials is not economical in most cases and seeking new efficient way of achieving low sulfur fuel is therefore desirable to date.
Unprecedentedly,using O2 as oxidant,our present work developed a new and green POM@MOFs catalyzed system to realize deep desulfurization of refractory sulfur compounds from fuel oil,which should provide a new pathway for efficient oxidative desulfurization under eco-sustainable conditions.
2. ExperimentalPreparation of the materials: The preparation of the catalysts was conducted according to the literature method [15]. The H3PW12O40,H3PMo12O40,H4SiW12O40,H7[P(W2O7)·(Mo2O7)6],threphthalic acid (H3BTC) and (CH3)4NOH were obtained commercially from Tianjin Kermel Chemical Reagent Co.,(Tianjin,China),Cu(NO3)2 was purchased from Tianjin Fuyu Fine Chemistry Co.,(Tianjin,China). Water was redistilled after deionisation. The heteropolyacid were dried under 230 °C for 3 h before use. The mixture of Cu(NO3)2 (0.96 g) and H3PMo12O40 (HPMo,0.8 g) for HPMo@MOFs,H3PW12O40 (HPW,0.8 g) for HPW@MOFs,H7[P(W2O7)·(Mo2O7)6] (HPWMo,0.8 g) for HPWMo@MOFs and H4SiW12O40 (HSiW,0.8 g) for HSiW@MOFs in distilled water (40 mL) were stirred for 20 min,followed by the addition of H3BTC (0.84 g) and (CH3)4NOH (0.36 g),respectively. After that,continuous stirring of the mixture was performed for about 30 min at r.t. The turbid mixture (pH 2-3) was sealed in a Teflon-lined autoclave and heated at 180 °C for 24 h,and then was slowly cooled to room temperature. Blue or green crystals were then collected.
The O2 driven oxidative desulfurization process: A certain amount (0.123 g) of dibenzothiophene (DBT,99%) was dissolved in 60 mL octane contained in a three-necked flask to obtain the simulated oil with 500 mg/L (500 ppm) sulfur content,60 mL distilled water was then added as extraction agent. Using thermostate water bath to stir the mixture at 90 °C for 10 min,then oxygen was bubbled into the mixed liquid (the flow rate of O2 was 90 mL/min),followed by the addition of catalyst. Catalyst dosage is 1% of the mass of normal octane (0.421 g). Reaction liquids in octane phase were sampled,respectively at a series of reflux condensation reaction time within 180 min and analyzed afterwards with a micro coulometer (WK-2E).
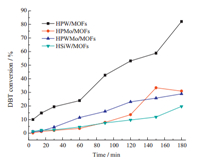
3. Results and discussionThe results are shown in Fig. 1. During the catalytic ODS process,under the same condition,the efficiencies of oxidative desulfurization decrease in the order of HPW@MOFs > HPMo@- HPMo@MOFs > HPWMo@MOFs > HSiW@MOFs. The DBT removal rate can reach 83% in 180 min with HPW@MOFs. The result is influenced by the Brønsted acidity sites on POMs. It is important to protect the acid sites to allow the reactant molecules access the acidic sites on POMs,which is responsible for their highly catalytic activity. HPW@MOFs shows a higher catalytic activity than other catalysts,which is attributed to H3PW12O40 (HPW) species’ strongest Brønsted acid among the Keggin series [16]. From Fig. 1,the desulfurization reaction over HPW@MOFs shows a rising trend in 180 min. Therefore we prolonged the reaction time to 240 min in subsequent experiments and investigated the effects of oxygen flow rate and the amount of catalyst on the DBT conversion over HPW@MOFs.
|
Download:
|
| Fig. 1. Oxidative desulfurization effects of the composite catalysts from different polyoxometalates and same MOFs, using O2 as oxidant. Conditions: reaction temperature, 90 °C; the flow rate of O2, 90 mL/min; catalyst dosage; 1% the mass of normal octane; extracting agent, distilled water; reaction time, 180 min. | |
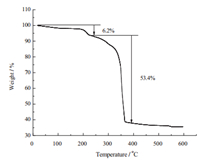
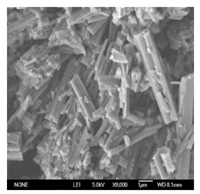
Characterizations of the HPW@MOFs were conducted by XRD and IR to confirm the structures of HPW@MOFs. Comparison of the HPW and HPW@MOFs powder XRD patterns (Fig. 2). There are several peaks which emerge mainly at 6°,9°,12°,17°,23°,24°,26° and 28°,showing that the polyoxometalate anion was retained after immobilization. The strongest peak appears at 6°,according to the literature [16, 17],the reflection at 2θ = 6° is a typical feature of the Keggin structure. The IR spectrum (Fig. 3) contains bands at 800-1100 cm-1,which matches those exhibited by heteropolyacid. The HPW anion contains the typical bands [18] of W-Oc-W at 813 cm-1,W-Ob-W at 893 cm-1,W=Od at 981 cm-1,P-Oa at 1079 cm-1. The XRD and IR characterizations of the HPW@MOFs confirm the presence of HPW in the cavities of the crystalline skeleton support,which is in line with the previous literature [15]. From the TG analysis (Fig. 4),an obvious plateau of 200- 250 °C can be observed for HPW@MOFs,indicating its high thermal stability. HPW@MOFs have tunable pore structure and show high BET surface area as up to 1102 m2/g measured with America Tristar 3020 physisorption apparatus. The SEM image (Fig. 5) of HPW@MOFs shows that the composite material is formed by irregular crystals with strip shape.

|
Download:
|
| Fig. 2. Powder XRD patterns of pure HPW and HPW@MOFs. | |

|
Download:
|
| Fig. 3. IR spectra of HPW@MOFs. | |
|
Download:
|
| Fig. 4. The thermogravimetric (TG) analysis curve of HPW@MOFs. | |
|
Download:
|
| Fig. 5. SEM image of HPW@MOFs. | |
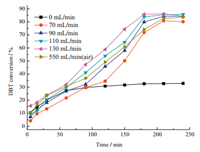
Fig. 6 presents the influence of the oxidant flow rate on the DBT conversion over HPW@MOFs. Under the same condition,the efficiency of the oxidative desulfurization is far superior to nonoxidative desulfurization,especially when the O2 flow rate was 110 mL/min,the best desulfurization rate can reach 86% within 240 min. The desulfurization efficiency increases with O2 flow rate within 210 min. However,in the next 30 min,the DBT conversion decreased from 86% to 84%. It was more apparent when O2 flow rate was 130 mL/min. The reason may be that the shortened contact time of O2 with DBT and the increased volatilization rate of solvent lead to the decrease of DBT conversion. Considering the desulfurization efficiency and economic factors,the 110 mL/min O2 flow rate was chosen as the optimum. Both activities of Cu2+ clusters and Keggin polyanion were supposed to contribute to the positive results. The reaction process was proposed as: (1) Copper and oxygen molecules forms activated complex Cu-O2 which could promote the oxidation process of DBT. Besides,the Cu2+ ions have a strong interaction with the sulfur atom from organosulfur compounds,and Cu-MOF is constructed by a multi-carboxylate with large conjugated aromatic rings [19],which aid the adsorption of organosulfur compounds. (2) Tungsten active sites in polyacid can accelerate the activation of oxygen. Oxygen interacts with heteropolyacid to form peracid which has strong oxidant ability,and the latter can easily oxidize dibenzothiophene to sulfone.
|
Download:
|
| Fig. 6. The effect of air and O2 flow rate on DBT removal rate with HPW@MOFs.Conditions: reaction temperature, 90 °C; catalyst dosage, 1% the mass of normal octane; extracting agent, distilled water; reaction time, 240 min. | |
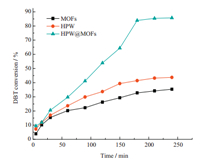
As can be seen in Fig. 7,the pure MOFs show a 35% DBT conversion in 240 min. The pure MOFs give high DBT conversion than POM@MOFs,because of the rich porous structure and higher specific surface area of pure MOFs. The oxidative desulfurization reaction using HPW as catalyst is more effective than MOFs,and a 44% DBT conversion was achieved in 240 min. It could be attributed to the activation of oxidant on tungsten active sites in polyacid. However,compared with the performance of pure HPW,the HPW@MOFs is more effective,which is due to the stronger interaction between the sulfur atom of DBT with copper ions,and the activation of oxidant on copper active sites [20]. In order to achieve economical desulfurization,in substitution of the foregoing O2,air was utilized as oxidant (Fig. 6). To ensure the actual O2 feed rate equivalent to 110 mL/min O2,the air flow rate was set as 550 mL/min. After 240 min,84% of the DBT was oxidized and removed from model oil. Hence,it is practically feasible and really more economical to use air as oxidant in desulfurization.
|
Download:
|
| Fig. 7. Comparison of oxidative desulfurization effect of DBT with HPW@MOFs, HPW and MOFs. Conditions: reaction temperature, 90 °C; the flow rate of O2, 110 mL/min; catalyst dosage; 1% the mass of normal octane; extracting agent, distilled water; reaction time, 240 min. | |
The effect of HPW@MOFs dosage on sulfur removal is shown in Fig. 8,the results indicate that it is difficult to achieve deep sulfur level without catalyst. The desulfurization efficiency increases with the dosage of catalyst. When the dosage of the catalyst is increased to 3% the mass of normal octane,the efficiency of the DBT conversion reaches 90% after 240 min.

|
Download:
|
| Fig. 8. The influence of HPW@MOFs dosage on oxidative desulfurization. Conditions: reaction temperature, 90 °C; the flow rate of O2, 110 mL/min; extracting agent, distilled water; reaction time, 240 min. | |
Besides,the recyclability of the HPW@MOFs was investigated by performing consecutive cycles of the foregoing ODS process. The catalyst was recovered at the end of each reaction by vacuum suction filter and washed with distilled water at room temperature,and then was put in oven at 120 °C to dry for 24 h. The obtained solid was reused as catalyst in a next fresh ODS process under the same experimental condition. The catalytic activity of HPW@MOFs maintains quite stable after three cycles with a slight decrease (3%) of sulfur removal rate. The very minor loss of efficiency of the ODS process is probably due to the leaching loss of the catalyst.
In view of the oxidative desulfurization performance achieved by HPW@MOFs,further experiments were carried out using real diesel. The sulfur contents in diesel samples were reduced from 225 mg/L to 28.37 mg/L with a sulfur conversion of 87% after 240 min under the following identical operating conditions: HPW@MOFs,1% the mass of real diesel; the flow rate of O2,110 mL/min; reaction temperature,90 °C; extracting agent,distilled water.
4. ConclusionIn summary,from the present study,we have found that the composite HPW@MOFs combined with oxidant O2 provides a very effective oxidative desulfurization system for the removal of dibenzothiophene from model oil and real diesel as well. 90% removal of DBT from model oil can be achieved after 240 min under optimal conditions. The catalyst can be used for three times with only a slight decline in activity. The investigation may broaden the scope of deep oxidative desulfurizaton with HPW@MOFs as catalyst and O2 as oxidant,to achieve an economical and highly efficient effect under mild conditions.
| [1] | X.L. Ma, K. Sakanishi, I. Mochida. Hydrodesulfurization reactivities of various sulfur compounds in diesel fuel. Ind. Eng. Chem. Res. 33 (1994) 218–222 |
| [2] | G.F. Zhang, R. Wan, F.L. Yu, H.X. Zhao. Clean fuel-oriented investigation of thiophene oxidation by hydrogen peroxide using polyoxometalate as catalyst. Chem. Pap. 63 (2009) 617–619 |
| [3] | W. Zhang, H. Zhang, J. Xiao, et al. Carbon nanotube catalysts for oxidative desulfurization of a model diesel fuel using molecular oxygen. Green Chem. 16 (2014) 211–220 |
| [4] | J.L. Wang, D.S. Zhao, K.X. Li. Oxidative desulfurization of dibenzothiophene using ozone and hydrogen peroxide in ionic liquid. Energy Fuels 24 (2010) 2527–2529 |
| [5] | L.T.L. Nguyen, C.V. Nguyen, G.H. Dang, K.K.A. Le, N.T.S. Phan. Towards applications of metal-organic frameworks in catalysis:Friedel-Crafts acylation reaction over IRMOF-8 as an efficient heterogeneous catalyst. J. Mol. Catal. A:Chem. 349 (2011) 28–35 |
| [6] | J.L.C. Rowsell, O.M. Yaghi. Strategies for hydrogen storage in metal-organic frameworks. Angew. Chem. Int. Ed. Engl. 44 (2005) 4670–4679 |
| [7] | X. Zhou, Y. Zhang, X.G. Yang, L.Z. Zhao, G.Y. Wang. Functionalized IRMOF-3 as efficient heterogeneous catalyst for the synthesis of cyclic carbonates. J. Mol. Catal. A:Chem. 361-362 (2012) 12–16 |
| [8] | L. Zhang, Y.H. Hu. Desorption of dimethylformamide from Zn4O(C8H4O4)3 framework. Appl. Surf. Sci. 257 (2011) 3392–3398 |
| [9] | X.F. Hu, Y.K. Lu, F.N. Dai, C.G. Liu, Y.Q. Liu. Host-guest synthesis and encapsulation of phosphotungstic acid in MIL-101 via "bottle around ship":an effective catalyst for oxidative desulfurization. Microporous Mesoporous Mater. 170 (2013) 36–44 |
| [10] | E. Rafiee, N. Nobakht. Keggin type heteropoly acid, encapsulated in metal-organic framework:a heterogeneous and recyclable nanocatalyst for selective oxidation of sulfides and deep desulfurization of model fuels. J. Mol. Catal. A:Chem. 398 (2015) 17–25 |
| [11] | X.L. Ma, A.N. Zhou, C.S. Song. A novel method for oxidative desulfurization of liquid hydrocarbon fuels based on catalytic oxidation using molecular oxygen coupled with selective adsorption. Catal. Today 123 (2007) 276–284 |
| [12] | X.R. Zhou, J. Li, X.N. Wang, K. Jin, W. Ma. Oxidative desulfurization of dibenzothiophene based on molecular oxygen and iron phthalocyanine. Fuel Process. Technol. 90 (2009) 317–323 |
| [13] | J.T. Sampanthar, H. Xiao, J. Dou, et al. A novel oxidative desulfurization process to remove refractory sulfur compounds from diesel fuel. Appl. Catal. B:Environ. 63 (2006) 85–93 |
| [14] | S. Murata, K. Murata, K. Kidena, M. Nomura. A novel oxidative desulfurization system for diesel fuels with molecular oxygen in the presence of cobalt catalysts and aldehydes. Energy Fuels 18 (2004) 116–121 |
| [15] | C.Y. Sun, S.X. Liu, D.D. Liang, et al. Highly stable crystalline catalysts based on a microporous metal-organic framework and polyoxometalates. J. Am. Chem. Soc. 131 (2009) 1883–1888 |
| [16] | I.V. Kozhevnikov. Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem. Rev. 98 (1998) 171–198 |
| [17] | J.B. Moffat, J.B. McMonagle, D. Taylor. Microporous heteropoly oxometalate heterogeneous catalysis. Solid State Ionics 26 (1988) 101–108 |
| [18] | C.R. Deltcheff, M. Fournier, R. Franck. Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum(Ⅵ) and tungsten(Ⅵ) compounds related to the Keggin Structure. Inorg. Chem. 22 (1983) 207–216 |
| [19] | K.A. Cychosz, A.G. Wong-Foy, A.J. Matzger. Liquid phase adsorption by microporous coordination polymers:removal of organosulfur compounds. J. Am. Chem. Soc. 130 (2008) 6938–6939 |
| [20] | N.A. Khan, Z. Hasan, S.H. Jhung. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs):a review. J. Hazard. Mater. 244-245 (2013) 444–456 |
 2016, Vol. 27
2016, Vol. 27 


