b College of Life Science, Perking University, Beijing 100871, China
The development of photoswitches provides enormous potential for applications in chemistry, biology and material science [1, 2, 3, 4, 5, 6, 7]. With an increasing number of examples, photopharmacology is rapidly developing recently [8, 9]. The photocontrol of the bioactivity has many advantages over the conventional method, such as precise manipulation of activity of interest, antiresistance and avoidance of side effects. Thus, many photoswitchable pharmaceuticals have been elaborately designed and achieved by attaching a light-responsive moiety to a bioactive molecules, such as using an optical mechanism to control antibacterial activity [10], coupling a photoswitch with the propofol analogue [11, 12], optical control of insulin release with photoswitchable sulfonylurea [13], photoswitchable acetylcholinesterase inhibitors [14], nociception regulators [15], mast cell activation inhibitors [16] and microtubule formation inhibitors [17].
The optical control of activity was well addressed in the pharmaceutical area but seldom used in pesticide design. We recently reported a first example of photoswitchable neonicotinoids by merging imidacloprid (IMI) with azobenzene, which facilitated the remote regulation of insecticide performance with light [18]. Activity variation upon irradiation was achieved in the above examples, but the difference was not large enough and the relatively poor solubility limited their applications.
The azobenzene is a most commonly used photoswitched motif, but it has poor solubility in water. The recently developed azopyridines (AP) revealed a novel type of photoswitchable molecules with excellent properties, such as quantitative rates of photoisomerization, good water solubility and slow thermal isomerization [19, 20].
Enlightened by the above descriptions, therefore, to develop a novel photoswitchable version of imidacloprid, our strategy here is trying to replace the chloropyrindyl part with AP by sharing a common pyridine fragments, generating the target compounds (Fig. 1). Besides, we hope the introduction of the AP fragment would lead to the improvement of the water solubility and insecticidal activity.

|
Download:
|
| Fig. 1. Molecular design of azopyridine-imidacloprid as photoswitchable neonicotinoids. | |
2. Experimental 2.1. Chemicals and instrumentations
Melting points were recorded on a Büchi B540 apparatus (Büchi Labortechnik AG, Flawil, Switzerland) and are uncorrected. 1H NMR and 13C NMR spectra were recorded on a Bruker AM-400 (400 MHz) spectrometer with CDCl3 or DMSO-d6 as the solvent and TMS as the internal standard. Chemical shifts are reported in δ (parts per million) values. Electrospray ionization (ESI) mass spectrometry was performed in an HP 1100 LC-MS spectrometer. Analytical thinlayer chromatography (TLC) was carried out on precoated plates (silica gel 60 F254), and spots were visualized under ultraviolet (UV) light. Column chromatography was performed using 200-300 mesh silica gel (Hailang, Qingdao). The water solubility of the compounds was determined by SiriusT3 (Sirius Analytical Ltd, UK). Unless otherwise noted, reagents and solvents were used as received from commercial suppliers. Yields were not optimized. All reactions were carried out under a protective atmosphere of drying nitrogen or utilizing a calcium chloride tube.
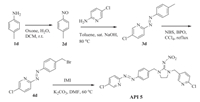
2.2. Synthesis the target compoundsCompounds API 1-API 4 were synthesized starting from anilines (Scheme 1). Oxidation of anilines by Oxone generated nitrosobenzenes, which then reacted with 2-amino-5-methylpyridine to construct the corresponding azopyridine. Bromination of azopyridine by NBS/BPO afforded bromomethyl-intermediate, which coupled with N-(imidazolidin-2-ylidene)nitramide or IMI to provide the final products (Scheme 1). API 5 was prepared by the similar procedure from p-toluidine and 2-amino-5-chloropyridine (Scheme 2). The synthetic procedure of API 1 as representative is given as follow and the detailed syntheses for API 2-5 are provided in the Supporting information.

|
Download:
|
| Scheme. 1. Synthesis of API 1–API 4. | |
|
Download:
|
| Scheme. 2. Synthesis of API 5. | |
Synthesis of methyl-2-phenyldiazenylpyridine (3a): Aniline (1a) (33.1 mmol, 1.0 equiv.) was dissolved in 100 mL of dichloromethane. To this solution was added potassium peroxymonosulfate (Oxone) (66.2 mmol, 2.0 equiv.) in 400 mL of water. The solution was stirred under nitrogen at room temperature until TLC monitoring indicated the complete consumption of the starting material (0.5 h). After separation of the layers, the aqueous layer was extracted with dichloromethane twice. The combined organic layers were washed with 1 mol/L HCl, saturated sodium bicarbonate solution, water, brine, dried (magnesium sulfate) and evaporated to dryness affording the crude nitrosobenzene (2a). Nitrosobenzene is directly used for the next step without further purification. Crude nitrosobenzene (3.49 mmol, 1.2 equiv.) was dissolved in 25 mL of toluene. To this solution was added 2-amino- 5-methylpyridine (2.9 mmol, 1.0 equiv.). Then to this solution was added saturated aqueous solution of sodium hydroxide (12 mmol, 4.0 equiv.). The resulting mixture was stirred at 60 ℃ for 30 min. Then 15 mL of water was added in the mixture, after separation of the layers, the aqueous layer was extracted with ethyl acetate twice. The combined organic layers were washed with brine, dried (magnesium sulfate) and evaporated to dryness. Purification by chromatography (petroleum ether/ethyl acetate = 10:1, silica gel) yielded the product as an orange solid. Yield 43%, 1H NMR (400 MHz, CDCl3): δ 8.64 (d, 1H, J = 2.0 Hz), 8.08-8.01 (m, 2H), 7.87 (dd, 1H, J = 8.2, 2.3 Hz), 7.72 (d, 1H, J = 8.2 Hz), 7.57-7.52 (m, 3H), 2.54 (s, 2H).
Synthesis of 5-bromomethyl-phenyldiazenylpyridine (4a): 3a (10 mmol, 1.0 equiv.) was dissolved in 25 mL of carbon tetrachloride. To this solution was added benzoyl peroxide (BPO) (1 mmol, 0.1 equiv.). Then N-bromosuccinimide (NBS) (11 mmol, 1.1 equiv.) was partially added to this solution. The solution was stirred under nitrogen at 70 ℃ for 12 h. The precipitate was separated by filtration; the filtrate was washed with brine, dried (magnesium sulfate) and evaporated to dryness. Purification by chromatography (petroleum ether/ethyl acetate = 20:1, silica gel) afforded the pure product as an orange solid. Compound 4a: Yield 73%, 1H NMR (400 MHz, CDCl3): δ 8.74 (d, 1H, J = 2.0 Hz), 8.08-8.01 (m, 2H), 7.94 (dd, 1H, J = 8.2, 2.3 Hz), 7.82 (d, 1H, J = 8.2 Hz), 7.57-7.52 (m, 3H), 4.55 (s, 2H).
Synthesis of N-(1-((6-((E)-(2-bromophenyl)diazenyl)pyridin- 3-yl)methyl)imidazolidin-2-ylidene)nitramide (API 1): N-nitroiminoimidazolidine (14 mmol, 1.4 equiv.) and K2CO3 (14 mmol, 1.4 equiv.) were added to DMF (5 mL) and the mixture was stirred for 10 min, then compound 4a (10 mmol, 1.0 equiv.) was added to this solution. The resulting mixture was stirred at 60 ℃ under Ar for 8 h. The precipitate was separated by filtration. The solvent was removed in vacuo. Purification by chromatography (dichloromethane/ethyl acetate = 3:1, silica gel) yielded API 1 as an orange solid. Yield 56%, m.p. 153.0-154.3 ℃; 1H NMR (400 MHz, DMSO-d6): δ 9.00 (s, 1H), 8.67 (d, 1H, J = 1.9 Hz), 8.03-7.92 (m, 3H), 7.75 (d, 1H, J = 8.2 Hz), 7.67-7.62 (m, 3H), 4.60 (s, 2H), 3.71-3.62 (m, 2H), 3.59-3.52 (m, 2H); 13C NMR (101 MHz, DMSO-d6): δ 162.27, 160.39, 151.83, 148.75, 138.24, 134.16, 132.49, 129.60, 122.94, 113.35, 45.16, 44.99, 41.61, HRMS (ESI): m/z calcd. for C15H15N7O2 [M+Na]+ 324.1185, found 348.1186.
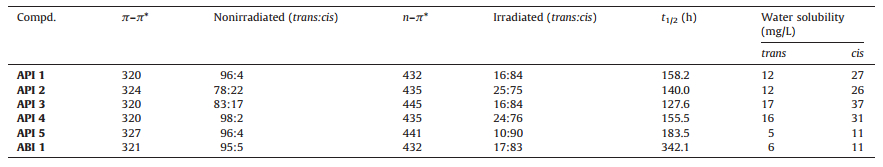
2.3. PhotoisomerizationAbsorption spectra were recorded on a Lambda 25 UV/vis spectrometer (PerkinElmer, Shanghai). Target compounds were dissolved in CH3CN using a microcuvette. Photochromism is irradiated by a hand-held MQK-WFH-204B ultraviolet lamp (365 nm, 10 mW /cm2, MQK, Shanghai). UV-vis absorption spectroscopy was used to detect the change of absorption profiles of target compounds upon 365 nm irradiation. The isomerization of the API 1 and API 4 analogues was also investigated in detail by 1H NMR spectroscopy. The corresponding UV-vis and 1H NMR spectra were provided in supporting information. The proportions of trans/cis isomer at the photostationary states before and after irradiation were determined by HPLC analysis. The trans/cis ratios were calculated at the isosbestic points. The half-lives of thermal relaxations under dark condition are shown in Table 1.
|
|
Table 1 Maximum absorbance wavelength (nm), ratio of trans and cis isomers, the rate of thermal relaxation and the water solubility of APIs. |
2.4. Insecticidal activity assay
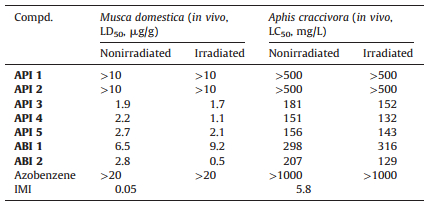
Insecticidal test of APIs was done with cowpea aphids (Aphis craccivora). The plant leaves of horsebean with about 50 apterous adults were dipped in corresponding APIs solutions containing Triton X-100 (0.1 mg/L) for 5 s and the excess solution was sucked out with a filter paper. Then burgeons were positioned in the conditioned room (25 ± 1 ℃). Water with Triton X-100 (0.1 mg/L) was used as control. Twenty-four hours after treatment, the mortality rate was measured. Each treatmenthad three repetitionsand the data were subjected to probit analysis. Insecticidal test for house fly (Musca domestica). House fly adults were anesthetized with carbon dioxide for 10 min and then were treated with the APIs dissolved in distilled water or 10% DMSO aqueous solution by intrathoracic injection (0.4 μL for each fly). Twenty flies were treated for each dosage with duplicate samples. The mortality rate was measured 24 h after treatment. Each treatment had three repetitions and the data were subjected to probit analysis.
3. Results and discussionPreviously, we merged the IMI with azobenzene to successfully generate photoswitchable insecticidal molecules. Here, a similar strategy was employed using azopyridine as a replacement. Imidacloprid was a successful neonicotinoid in the past decades withannual salesapproachingto one billionUS dollars [21, 22].The SAR study indicated that the pyridine is an indispensable pharmacophore by interacting with the nicotinic acetylcholine receptors through cation-π interactions [23]. Thus, we incorporatedtheazopyridine intotheIMI bysharing acommonpyridineor into the imidazoline ring of IMI to generate the photoswitchable azopyridine-imidacloprid (API) derivatives.
The isomerization of the API analogues was investigated in detail using 1H NMR spectroscopy, UV-vis spectroscopy and HPLC analysis. The trans-isomers showed a typical azobenzene absorption at around 325 nm (319-330 nm) belonging to the π-π* transition band. Irradiation with 365 nm UV light isomerizes the azopyridine part to its metastable cis-form with the appearance of absorption at around 430 nm corresponding to n-π* transition band, indicating the initiation of trans-to-cis photoisomerization (Fig. 2). The trans/cis content was determined by HPLC analysis at the photostationary state. The trans-to-cis isomerization showed high transformation rate of photoisomerization ranging from 24:76 to 10:90. The cis-to-trans change can be achieved upon irradiation at 430 nm and the half-life of the thermal relaxation of the cis-isomer was more than 120 h, which guaranteed the stability of cis-isomers for biological test.

|
Download:
|
| Fig. 2. UV–vis spectrum of API 1 and API 5 upon irradiation at 365 nm at varied times. | |
Then the water solubility of the compounds was studied. All the compounds except API 5 have higher solubility than the azobenzene analogue ABI 1. API 5 had very poor water solubility as a trans-isomer, indicating that appending azopyridine in such a way was unfavourable to the solubility. Interestingly, almost a 2-fold solubility increase was observed for compound API 1-API 5 and ABI 1 upon irradiation, suggesting that the bend form of azopyridine would enhance the solubility (Table 1). Pyridine fragment in the AP is a hydrophilic group in comparison with benzene in the azobenzene, which leads to the higher water solubility of azopyridine-imidacloprid analogues.
Having identified the suitable photochemical properties, the insecticidal performance of these novel photoresponsive insecticides was evaluated against house fly (Musca domestica) by the intrathoracic injection and cowpea aphid (Aphis craccivora) by the leaf-dip method, respectively. The results are summarized in Table 2. API 1 and API 2 showed very low insecticidal efficacy both to Musca and cowpea aphid (LD50 > 10 μg/g and LC50 > 500 mg/L, respectively) for the two photoisomers. API 3, 4 and 5 exhibit almost the same level of activity to the two insects with LD50 values of around 2 μg/g for Musca and LC50 of 150-181 mg/L for aphid, respectively. 2-Chloropyridine is most important pharmacophore in the IMI, the removal of 2-chloropyridine will cause a sharp drop in insecticidal activity found in previous SAR study. We replaced the chloropyrindyl part with AP not only to increase the solubility in water but also wished to share a common pyridine fragment, so we synthesized API 1 and API 2. However, API 1 and API 2 showed very low insecticidal efficacy, possibly because AP is not a good pharmacophore as 2-chloropyridine. API 1 and 2 had no activity, possibly caused by the remove of 2-chloropyridine, while in compounds API 3-5, 2-chloropyridine were retained. To our disappointment, only slight activity difference was observed for API 3 before and after irradiation. In comparison with azobenzene analogues ABI 1, the API 3-5 showed nearly 3-fold activity increase along with increased solubility, upon irradiation, a 2-fold activity difference was achieved for API 4 and API 5. All the compounds had lower activity than the imidacloprid, indicating that such chemical modifications were not tolerated to the activity enhancement (Table 2).
|
|
Table 2 Insecticidal activity of APIs against house fly (Musca domestica) and cowpea aphid (Aphis craccivora). |
4. Conclusion
In conclusion, we prepared a novel type of photoswitchable imidacloprid derivatives by incorporating the photoisomerizable azopyridine unit based on our previous azobenzene-imidacloprid analogues. The azopyridine-imidacloprid analogues had higher insecticidal activity and water solubility than the corresponding azobenzene-imidacloprid analogues.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.03.033.
| [1] | P. Gorostiza, E. Isacoff. Optical switches and triggers for the manipulation of ion channels and pores. Mol. Biosyst. 3 (2007) 686–704 |
| [2] | F. Hamon, F. Djedaini-Pilard, F. Barbot, C. Len. Azobenzenes-synthesis and carbohydrate applications. Tetrahedron 65 (2009) 10105–10123 |
| [3] | C.W. Riggsbee, A. Deiters. Recent advances in the photochemical control of protein function. Trends Biotechnol. 28 (2010) 468–475 |
| [4] | T. Fehrentz, M. Schonberger, D. Trauner. Optochemical genetics. Angew. Chem. Int. Ed 50 (2011) 12156–12182 |
| [5] | W. Szymanski, J.M. Beierle, H.A. Kistemaker, W.A. Velema, B.L. Feringa. Reversible photocontrol of biological systems by the incorporation of molecular photoswitches. Chem. Rev. 113 (2013) 6114–6178 |
| [6] | M. Banghart, K. Borges, E. Isacoff, D. Trauner, R.H. Kramer. Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci. 7 (2004) 1381–1386 |
| [7] | S. Samanta, A.A. Beharry, O. Sadovski, et al. Photoswitching azo compounds in vivo with red light. J. Am. Chem. Soc. 135 (2013) 9777–9784 |
| [8] | W.A. Velema, W. Szymanski, B.L. Feringa. Photopharmacology:beyond proof of principle. J. Am. Chem. Soc. 136 (2014) 2178–2191 |
| [9] | J. Broichhagen, J.A. Frank, D. Trauner. A roadmap to success in photopharmacology. Acc. Chem. Res. 48 (2015) 1947–1960 |
| [10] | W.A. Velema, J.P. van der Berg, M.J. Hansen, et al. Optical control of antibacterial activity. Nat. Chem. 5 (2013) 924–928 |
| [11] | L. Yue, M. Pawlowski, S.S. Dellal, et al. Robust photoregulation of GABAA receptors by allosteric modulation with a propofol analogue. Nat. Commun 3 (2012) 1095 |
| [12] | M. Stein, S.J. Middendorp, V. Carta, et al. Azo-propofols:photochromic potentiators of GABA (A) receptors. Angew. Chem. Int. Ed. 51 (2012) 10500–10504 |
| [13] | J. Broichhagen, M. Schonberger, S.C. Cork, et al. Optical control of insulin release using a photoswitchable sulfonylurea. Nat. Commun 5 (2014) 5116 |
| [14] | X. Chen, S. Wehle, N. Kuzmanovic, et al. Acetylcholinesterase inhibitors with photoswitchable inhibition of beta-amyloid aggregation. ACS Chem. Neurosci. 5 (2014) 377–389 |
| [15] | A. Mourot, T. Fehrentz, Y. Le Feuvre, et al. Rapid optical control of nociception with an ion-channel photoswitch. Nat. Methods 9 (2012) 396–402 |
| [16] | W.A. Velema, M. van der Toorn, W. Szymanski, B.L. Feringa. Design, synthesis, and inhibitory activity of potent, photoswitchable mast cell activation inhibitors. J Med. Chem. 56 (2013) 4456–4464 |
| [17] | M. Borowiak, W. Nahaboo, M. Reynders, et al. Photoswitchable inhibitors of microtubule dynamics optically control mitosis and cell death. Cell 162 (2015) 403–411 |
| [18] | Z. Xu, L. Shi, D. Jiang, et al. Azobenzene modified imidacloprid derivatives as photoswitchable insecticides:steering molecular activity in a controllable manner. Sci. Rep 5 (2015) 13962 |
| [19] | J. Garcia-Amoros, M. Diaz-Lobo, S. Nonell, D. Velasco. Fastest thermal isomerization of an azobenzene for nanosecond photoswitching applications under physiological conditions. Angew. Chem. Int. Ed. 51 (2012) 12820–12823 |
| [20] | J. Garcia-Amoros, E. Gomez, E. Valles, D. Velasco. Photo-controllable electronic switches based on azopyridine derivatives. Chem. Commun. 48 (2012) 9080–9082 |
| [21] | P. Jeschke, R. Nauen, M.E. Beck. Nicotinic acetylcholine receptor agonists:a milestone for modern crop protection. Angew. Chem. Int. Ed. 52 (2013) 9464–9485 |
| [22] | J.E. Casida. Curious about pesticide action. J. Agric. Food Chem. 59 (2011) 2762–2769 |
| [23] | S. Kagabu. Discovery of imidacloprid and further developments from strategic molecular designs. J. Agric. Food Chem. 59 (2011) 2887–2896 |
 2016, Vol. 27
2016, Vol. 27