b State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
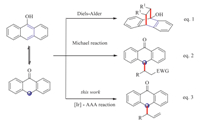
Anthrones and their derivatives play an important role in medicinal chemistry and organic synthesis [1]. Many anthrone derivatives exhibit interesting biological activity, such as antimicrobial, antipsoriatic, telomerase inhibiting, and antitumor activity [2]. Over the past several years, considerable efforts have been devoted to the construction of anthrone derivatives in an enantioselective manner. In this regard, 9-anthrol, the enol tautomer of anthrone, has been generally used as a diene component in asymmetric Diels-Alder cycloaddition reactions with a variety of dienophiles (Scheme 1, eq. 1) [3]. For instance, in 2006, Tan and coworkers demonstrated a highly enantioselective Diels-Alder reaction of anthrones and phenylmaleimides with chiral bicyclic guanidine as catalyst, giving bridged Diels-Alder cycloadducts in excellent yields and enantioselectivity [3f]. In addition, anthrone could also serve as a nucleophile at the C10 position exemplified by the Michael addition to α, β-unsaturated carbonyls [4], and nitroalkenes [5] (Scheme 1, eq. 2). Despite of the fact that remarkable progresses have been made toward various transformations of anthrones by organocatalysis, little attention has been paid on transition-metal catalyzed asymmetric reactions of anthrones. The chemoselectivity between O- and C-atom is considered as a main challenge in this reaction due to the equilibrium between anthrol and anthrone [6]. Thus the development of an efficient chemo- and enantioselective reaction is highly desirable for construction of valuable functionalized anthrones in both academic and industrial laboratories. With our continuing interest in allylic substitution reactions [7, 8], we recently found that anthrones are suitable carbon-nucleophiles in Ir-catalyzed asymmetric allylic substitution reaction (Scheme 1, eq. 3). Herein we report our preliminary results from this study.
|
Download:
|
| Scheme. 1. Catalytic asymmetric reactions of anthrone. | |
2. Experimental
General procedure for iridium-catalyzed enantioselective allylic alkylation: A flame-dried Schlenk tube was cooled to room temperature and filled with argon. To this flask were added [Ir(COD)Cl]2 (5.7 mg, 0.01 mmol, 2 mol%), phosphoramidite ligand BHPphos L4 (13.5 mg, 0.02 mmol, 4 mol%), THF (0.5 mL) and n-propylamine (0.5 mL). The reaction mixture was heated at 50 ℃ for 30 min and then the volatile solvents were removed in vacuo to give a pale yellow solid. After that, a solution of allylic carbonate 1 (0.55 mmol, 110 mol%) and anthrone (0.50 mmol) in 3.0 mL CH2Cl2, and t-BuOLi (40 mg, 0.50 mmol, 100 mol%) were added. After the reaction was complete (monitored by TLC), the crude reaction mixture was filtrated with celite and washed with EtOAc. The solvents were removed under reduced pressure. The ratio of branched and linear product was determined by 1H NMR of the crude reaction mixture. Then the residue was purified by silica gel column chromatography (PE/acetone = 20/1 to 10/1) to afford the desired product 3. The characterization data of the products are summarized in the Supporting information.
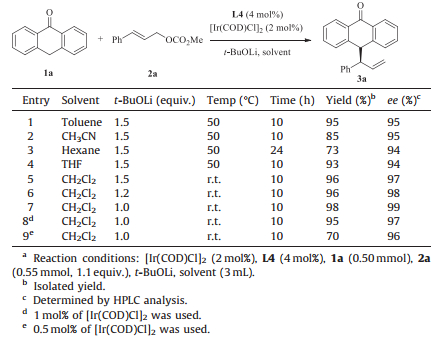
3. Results and discussionAt the outset of our study, we chose cinnamyl methyl carbonate (2a) as the electrophile to test the allylic substitution reaction of anthrone 1a. In the presence of an iridium catalyst prepared from [Ir(COD)Cl]2 (2 mol%) and (S, S, Sa)-L1 (4 mol%) [9], the allylic alkylation reaction of anthrone with 1.5 equiv. of t-BuOLi as base in toluene proceeded smoothly to give the C10 allyl substituted product exclusively in 73% yield and 89% ee (Table 1, entry 1). The reaction with the Alexakis ligand (L2) gave the product in a better yield (93%) but with slightly lower ee (80%) (Table 1, entry 2). The reactions with THQphos (L3) and N-arylhydrylaniline derived phosphoramidites (L4, L5) developed in our group [10] afforded excellent results. BHPphos (L4) was found to be the most efficient ligand, affording 3a in 95% yield and 95% ee (Table 1, entry 4). When the scaffold of the ligand was switched from BINOL to SPINOL, good yield was obtained with decreased enantioselectivity (Table 1, entry 6, 90% yield, -79% ee). It is remarkable that almost no O-allylation product and only trace amount of linear allylation product were observed in all cases. Encouraged by these preliminary results, we subsequently examined the effect of bases in this reaction. These results in Table 1 indicated that t-BuOLi (Table 1, entry 4) was the most suitable one among the bases tested for the present reaction (Table 1, entries 7-11). When no external base was added, the reaction was much slower, providing 3a in 50% yield and 92% ee (Table 1, entry 12).
|
|
Table 1 Screening of ligands and bases.a |
Under the above conditions (Table 1, entry 4), several solvents were further evaluated. The results are summarized in Table 2. The reaction proceeded to complete in CH2Cl2 at room temperature, giving the desired product in almost quantitative yield with the best enantioselectivity (97% ee) (Table 2, entry 5 vs. entries 1-4). A survey of the loading of the base and catalyst revealed that 1 equiv. of t-BuOLi and 2 mol% of [Ir(COD)Cl]2 provided the best results (Table 2, entry 7, 98% yield, 99% ee). There was a decrease in yield when the catalyst loading was further reduced to 0.5 mol% (Table 2, entry 9, 70% yield, 96% ee).
|
|
Table 2 Screening of solvents and catalyst loading.a |
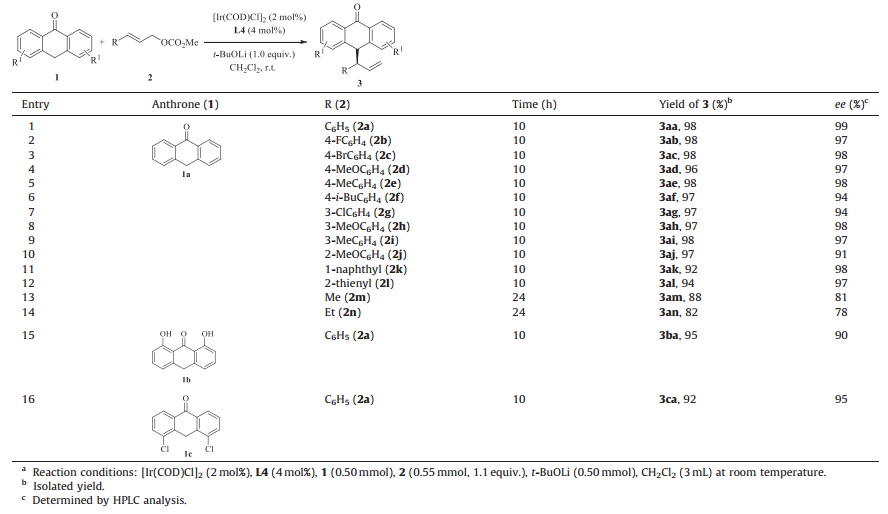
With the optimized conditions in hand, we investigated the substrate scope of this allylic substitution reaction. We are pleased to find that cinnamyl carbonates bearing an either electrondeficient or electron-donating group (4-F, 4-Br, 4-OMe, 4-Me, 4-i-Bu, 3-Cl, 3-OMe, 3-Me, 2-OMe, Table 3, entries 2-10) could exclusively give the branched allyl substituted products in excellent yields and enantioselectivity (96%-98% yields, 91%-98% ee). The ortho methoxy substituted cinnamyl carbonate could also provide the desired product (3aj) in 97% yield and 91% ee (Table 3, entry 10). 1-Naphthyl and 2-thienyl cinnamyl carbonates (2k-2l) were also tolerated, affording products in 92% yield, 98% ee (entry 11) and 94% yield, 97% ee (entry 12), respectively. Alkylallyl carbonates (Me, Et) could occur smoothly, but with slightly decreased ee values (entries 13-14, 88% yield, 81% ee; 82% yield, 78% ee). It is notable that anthrones bearing varied substituents could also be tolerated. The reactions with 4, 5-dichloroanthrone and 1, 8-dihydroxy-9-[10H]-anthracenone gave their corresponding products in 95% yield, 90% ee (entry 15) and 92% yield, 95% ee (entry 16), respectively. The absolute configuration of the products was determined by VCD calculation of product 3ae (98% ee) as R (see the Supporting Information for details).
|
|
Table 3 Substrate scope.a |
The terminal double bond of the products provides a platform for further transformation. For instance, the terminal alkenyl group of allylic substituted anthrone 3aa could be transformed into primary alcohol 4aa by hydroboration and subsequent oxidation. This procedure worked well to afford product 4aa in 63% yield and 97% ee (Scheme 2) [11].

|
Download:
|
| Scheme. 2. Transformation of product 3aa. | |
4. Conclusion
In summary, we have realized the enantioselective allylic substitution reaction of anthrones by using an Iridium catalyst derived from Ir(COD)Cl]2 and BHPphos. The reaction proceeds under very mild reaction conditions, and can provide various substituted 10-allylanthrones in excellent yields with excellent chemo-, regio- and enantioselectivity.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.02.017.
| [1] | S. Coffey, Rodd's Chemistry of Carbon Compounds, vol. Ⅲ, Part H, Elsevier, New York, 1979. |
| [2] |
(a) D.W. Cameron, C.E. Skene, Synthesis of the androgen-receptor antagonists (±)-Ws9761 A and B, Aust. J. Chem. 49(1996) 617-624; (b) K. Müller, H. Prinz, Antipsoriatic anthrones with modulated redox properties. 4. Synthesis and biological activity of novel 9,10-dihydro-1,8-dihydroxy-9-oxo-2-anthracenecarboxylic and-hydroxamic acids, J. Med. Chem. 40(1997) 2780-2787; (c) T. Pecere, M.V. Gazzola, C. Mucignat, et al., Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors, Cancer Res. 60(2000) 2800-2804; (d) K.Müller, Pharmaceutically relevant metabolites fromlichens, Appl. Microbiol. Biotechnol. 56(2001) 9-16; (e) H.S. Huang, J.M. Hwang, Y.M. Jen, et al., Studies on anthracenes. 1. Human telomerase inhibition and lipid peroxidation of 9-acyloxy 1,5-dichloroanthracene derivatives, Chem. Pharm. Bull. 49(2001) 969-973; (f) H. Prinz, Y. Ishii, T. Hirano, et al., Novel benzylidene-9(10H)-anthracenones as highly active antimicrotubule agents. Synthesis, antiproliferative activity, and inhibition of tubulin polymerization, J. Med. Chem. 46(2003) 3382-3394; (g) E. Hoffman, Cancer and the Search for Selective Biochemical Inhibitors, 2nd ed., CRC, Boca Raton, 2007; (h) A. Zuse, D. Schmidt, S. Baasner, et al., Sulfonate derivatives of naphtho[2,3-b]thiophen-4(9H)-one and 9(10H)-anthracenone as highly active antimicrotubule agents. Synthesis, antiproliferative activity, and inhibition of tubulin polymerization, J. Med. Chem. 50(2007) 6059-6066. |
| [3] |
For selected examples of asymmetric Diels-Alder reactions of anthrones, see:(a) O Riant, H.B. Kagan, L. Ricard, Asymmetric base-catalyzed cycloaddition between anthrone and some dienophiles, Tetrahedron 50(1994) 4543-4554; (b) K. Tokioka, S. Masuda, T. Fujii, Y. Hata, Y. Yamamoto, Asymmetric cycloaddition of anthrone with N-substituted maleimides with C2-chiral pyrrolidines, Tetrahedron:Asymmetry 8(1997) 101-107; (c) B. Peng, K. Cheng, D. Ma, Chiral guanidine catalyzed Michael addition reaction and Diels-Alder reaction of anthrone and N-methylmaleimide, Chin. J. Chem. 18(2000) 411-413;(d) K. Uemae, S. Masuda, Y. Yamamoto, Asymmetric cycloaddition of anthrone and maleimides catalyzed by C2-chiral pyrrolidines, J. Chem. Soc. Perkin Trans. 1(2001) 1002-1006; (e) R. Harrison, B. Rickborn, Synergistic catalysis of anthrone Diels-Alder reactions, Org. Lett. 4(2002) 1711-1713; (f) J. Shen, T.T. Nguyen, Y.P. Goh, et al., Chiral bicyclic guanidine-catalyzed enantioselective reactions of anthrones, J. Am. Chem. Soc. 128(2006) 13692-13693; (g) D. Akalay,G.Dürner,M.W.Göbel,Afirst case of asymmetric catalysis induced by metal-free bisoxazolines, Eur. J. Org. Chem. (2008) 2365-2368; (h) D. Akalay, G. Dürner, J.W. Bats, M.W. Göbel, C2-symmetric bisamidines:chiral Brønsted bases catalysing the Diels-Alder reaction of anthrones, Beilstein J. Org. Chem. 4(2008) 28; (i) A. Zea, G. Valero, A.N. Alba, A. Moyano, R. Rios, Bifunctional thiourea-catalyzed asymmetric addition of anthrones to maleimides, Adv. Synth. Catal. 352(2010) 1102-1106; (j) J.F. Bai, Y.L. Guo, L. Peng, et al., Enantioselective Diels-Alder reaction of anthrone and maleimide catalyzed by a simple chiral tertiary amine, Tetrahedron 69(2013) 1229-1233. |
| [4] |
(a) A.N. Alba, N. Bravo, A. Moyano, R. Rios, Enantioselective addition of anthrones to α,β-unsaturated aldehydes, Tetrahedron Lett. 50(2009) 3067-3069; (b) C. Wu, W. Li, J. Yang, X. Liang, J. Ye, Asymmetric organocatalytic Michael addition of anthrone to enone, Org. Biomol. Chem. 8(2010) 3244-3250. |
| [5] |
(a) M. Shi, Z.Y. Lei, M.X. Zhao, J.W. Shi, A highly efficient asymmetric Michael addition of anthrone to nitroalkenes with cinchona organocatalysts, Tetrahedron Lett. 48(2007) 5743-5746; (b) Y.H. Liao, H. Zhang, Z.J. Wu, et al., Enantioselective Michael addition of anthrone to nitroalkenes catalyzed by bifunctional thiourea-tertiary amines, Tetrahedron:Asymmetry 20(2009) 2397-2402; (c) T. He, X.Y. Wu, Enantioselective organocatalytic Michael addition of anthrone to nitroalkenes, Chin. J. Org. Chem. 9(2010) 1400-1404; (d) A. Zea, A.N. Alba, N. Bravo, A. Moyano, R. Rios, Asymmetric organocatalytic anthrone additions to activated alkenes, Tetrahedron 67(2011) 2513-2529; (e) H.W. Sun, Y.H. Liao, Z.J. Wu, et al., Enantioselective 1,6-Michael addition of anthrone to 3-methyl-4-nitro-5-alkenyl-isoxazoles catalyzed by bifunctional thiourea-tertiary amines, Tetrahedron 67(2011) 3991-3996. |
| [6] |
(a) M. Koerner, B. Rickborn, Anthrones as reactive dienes in Diels-Alder reactions, J. Org. Chem. 54(1989) 6-9; (b) H.G. Korth, P. Mulder, Anthrone and related hydroxyarenes:tautomerization and hydrogen bonding, J. Org. Chem. 78(2013) 7674-7682. |
| [7] |
For recent reviews on Ir-catalyzed allylic substitution reactions, see (a) H Miyabe, Y. Takemoto, Regio- and stereocontrolled palladium-or iridiumcatalyzed allylation, Synlett (2005) 1641-1655; (b) R. Takeuchi, S. Kezuka, Iridium-catalyzed formation of carbon-carbon and carbon-heteroatom bonds synthesis, 2006, 3349-3366; (c) G. Helmchen, A. Dahnz, P. Dübon, M. Schelwies, R. Weihofen, Iridium-catalysed asymmetric allylic substitutions, Chem. Commun. (2007) 675-691; (d) M. Stanley, J.F. Hartwig, Mechanistically driven development of iridium catalysts for asymmetric allylic substitution, Acc. Chem. Res. 43(2010) 1461-1475; (e) Z. Zhang, F. Xie, B. Yang, H. Yu,W. Zhang, Chiral phosphoramidite ligand and its application in asymmetric catalysis, Chin. J. Org. Chem. 31(2011) 429-442; (f) W.B. Liu, J.B. Xia, S.L. You, Iridium-catalyzed asymmetric allylic substitutions, Top. Organomet. Chem. 38(2012) 155-208; (g) P. Tosatti, A. Nelson, S.P. Marsden, Recent advances and applications of iridiumcatalysed asymmetric allylic substitution,Org. Biomol. Chem. 10(2012) 3147-3163; (h) C.X. Zhuo, C. Zheng, S.L. You, Transition-metal-catalyzed asymmetric allylic dearomatization reactions, Acc. Chem. Res. 47(2014) 2558-2573. |
| [8] |
For selected recent examples, see:(a) HHe, W.B. Liu, L.X. Dai, S.L. You, Enantioselective synthesis of 2,3-dihydro-1Hbenzo[b]azepines:iridium-catalyzed tandem allylic vinylation/amination reaction, Angew. Chem. Int. Ed. 49(2010) 1496-1499; (b) S. Zheng, N. Gao, W. Liu, et al., Regio- and enantioselective iridium-catalyzed allylation of thiophenol:synthesis of enantiopure allyl phenyl sulfides, Org. Lett. 12(2010) 4454-4457; (c) Q.F.Wu,W.B. Liu, C.X. Zhuo, et al., Iridium-catalyzed intramolecular asymmetric allylic dearomatization of phenols, Angew. Chem. Int. Ed. 50(2011) 4455-4458; (d) Q.F. Wu, C. Zheng, S.L. You, Enantioselective synthesis of spiro cyclopentane-1,3'-indoles and 2,3,4,9-tetrahydro-1H-carbazoles by iridium-catalyzed allylic dearomatization and stereospecific migration, Angew. Chem. Int. Ed. 51(2012) 1680-1683; (e) W.B. Liu, C.M. Reeves, S.C. Virgil, B.M. Stoltz, Construction of vicinal tertiary and all-carbon quaternary stereocenters via Ir-catalyzed regio-, diastereo-, and enantioselective allylic alkylation and applications in sequential Pd catalysis, J. Am. Chem. Soc. 135(2013) 10626-10629; (f) W.B. Liu, C.M. Reeves, B.M. Stoltz, Enantio-, diastereo-, and regioselective iridium-catalyzed asymmetric allylic alkylation of acyclic β-ketoesters, J. Am. Chem. Soc. 135(2013) 17298-17301; (g) M. Chen, J.F. Hartwig, Iridium-catalyzed enantioselective allylic substitution of unstabilized enolates derived from α,β-unsaturated ketones, Angew. Chem. Int. Ed. 53(2014) 8691-8695; (h) W. Chen, J.F. Hartwig, Cation control of diastereoselectivity in iridiumcatalyzed allylic substitutions. Formation of enantioenriched tertiary alcohols and thioethers by allylation of 5H-oxazol-4-ones and 5H-thiazol-4-ones, J. Am. Chem. Soc. 136(2014) 377-382; (i) J.Qu, L. Roßberg, G. Helmchen, Enantio- and regioselective iridium-catalyzed allylic esterification, J. Am. Chem. Soc. 136(2014) 1272-1275; (j) S. Krautwald, M.A. Schafroth, D. Sarlah, E.M. Carreira, Stereodivergent α-allylation of linear aldehydes with dual iridium and amine catalysis, J. Am. Chem. Soc. 136(2014) 3020-3023; (k) Z.P. Yang, Q.F.Wu, S.L. You, Direct asymmetric dearomatization of pyridines and pyrazines by iridium-catalyzed allylic amination reactions, Angew. Chem. Int. Ed. 53(2014) 6986-6989; (l) J.Y. Hamilton, D. Sarlah, E.M. Carreira, Iridium-catalyzed enantioselective allylic alkylation with functionalized organozinc bromides, Angew. Chem. Int. Ed. 54(2015) 7644-7647; (m) X. Zhang, Z.P. Yang, L. Huang, S.L. You, Highly regio- and enantioselective synthesis of N-substituted 2-pyridones:iridium-catalyzed intermolecular asymmetric allylic amination, Angew. Chem. Int. Ed. 54(2015) 1873-1876; (n) S. Breitler, E.M. Carreira, Formaldehyde N,N-dialkylhydrazones as neutral formyl anion equivalents in iridium-catalyzed asymmetric allylic substitution, J. Am. Chem. Soc. 137(2015) 5296-5299; (o) C.X. Zhuo, Y. Zhou, Q. Cheng, L. Huang, S.L. You, Enantioselective construction of spiroindolines with three contiguous stereogenic centers and chiral tryptamine derivatives via reactive spiroindolenine intermediates, Angew. Chem. Int. Ed. 54(2015) 14146-14149; (p) Z.P. Yang, Q.F. Wu, W. Shao, S.L. You, Iridium-catalyzed intramolecular asymmetric allylic dearomatization reaction of pyridines, pyrazines, quinolines, and isoquinolines, J. Am. Chem. Soc. 137(2015) 15899-15906. |
| [9] | T.C. Kiener, C. Shu, C.D. Incarvito, J.F. Hartwig. Identification of an activated catalyst in the iridium-catalyzed allylic amination and etherification. Increased rates, scope, and selectivity. J. Am. Chem. Soc 125 (2003) 14272–14273 |
| [10] |
(a) W.B. Liu, H. He, L.X. Dai, S.L. You, Synthesis of 2-methylindoline- and 2-methyl-1,2,3,4-tetrahydroquinoline-derived phosphoramidites and their applications in iridium-catalyzed allylic alkylation of indoles, Synthesis (2009) 2076-2082; (b) Q.F. Wu, H. He, W.B. Liu, S.L. You, Enantioselective construction of spiroindolenines by Ir-catalyzed allylic alkylation reactions, J. Am. Chem. Soc. 132(2010) 11418-11419; (c) J.B. Xia, C.X. Zhuo, S.L. You, Synthesis of cyclopropane-containing building blocks via Ir-catalyzed enantioselective allylic substitution reaction, Chin. J. Chem. 28(2010) 1525-1528; (d) C.X. Zhuo, W.B. Liu, Q.F. Wu, S.L. You, Asymmetric dearomatization of pyrroles via Ir-catalyzed allylic substitution reaction:enantioselective synthesis of spiro-2H-pyrroles, Chem. Sci. 3(2012) 205-208; (e) W.B. Liu, C. Zheng, C.X. Zhuo, L.X. Dai, S.L. You, Iridium-catalyzed allylic alkylation reaction with N-aryl phosphoramidite ligands:scope and mechanistic studies, J. Am. Chem. Soc. 134(2012) 4812-4821; (f) C.X. Zhuo, Q.F.Wu, Q. Zhao, Q.L. Xu, S.L. You, Enantioselective functionalization of indoles and pyrroles via an in situ-formed spiro intermediate, J. Am. Chem. Soc. 135(2013) 8169-8172; (g) Q.L. Xu, C.X. Zhuo, L.X. Dai, S.L. You, Highly enantioselective synthesis of tetrahydrocarbolines via iridium-catalyzed intramolecular Friedel-Crafts type allylic alkylation reactions, Org. Lett. 15(2013) 5909-5911; (h) X. Zhang, W.B. Liu, H.F. Tu, S.L. You, Ligand-enabled Ir-catalyzed intermolecular diastereoselective and enantioselective allylic alkylation of 3-substituted indoles, Chem. Sci. 6(2015) 4525-4529; (i) C.X. Zhuo, Q. Cheng,W.B. Liu, Q. Zhao, S.L. You, Enantioselective synthesis of pyrrole-based spiro- and polycyclic derivatives by iridium-catalyzed asymmetric allylic dearomatization and controllable migration reactions, Angew. Chem. Int. Ed. 54(2015) 8475-8479; (j) Z.L. Zhao, Q. Gu, X.Y. Wu, S.L. You, Pd(0)-catalyzed benzylation of indole through η3-benzyl palladium intermediate, Chin. J. Catal. 36(2015) 15-18; (k) Z.L. Zhao, Q.L. Xu, Q. Gu, X.Y. Wu, S.L. You, Enantioselective synthesis of 4-substituted tetrahydroisoquinolines via palladium-catalyzed intramolecular Friedel-Crafts type allylic alkylation of phenols, Org. Biomol. Chem. 13(2015) 3086-3092. |
| [11] | T.C. Morrill, C.A. D'Souza, L. Yang, A.J. Sampognaro. Transition-metal-promoted hydroboration of alkenes:a unique reversal of regioselectivity. J. Org. Chem 67 (2002) 2481–2484 |
 2016, Vol. 27
2016, Vol. 27