b Department of Chemistry, Beijing Normal University, Beijing 100050, China
During the past few decades, dendrimers have received increasing attention not only for their aesthetic attributes but also because of their wide range of applications in nanomedicine, molecular electronics, and catalysis [1, 2, 3, 4]. Among them, metallodendrimers represents an important class of dendrimers because the incorporation of metals provides the resulting metallodendrimers with the wider applications such as biological mimetics, supramolecular redox sensors, and light-harvesting antennae [5, 6, 7, 8]. Coordination-driven self-assembly [9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25], which is based onmetal-ligand coordination interaction, has provided a particularly powerful strategy for construction of supramolecular metallodendrimers with well-defined shapes and sizes. Although a variety of metallodendrimers have been prepared via coordination- driven self-assembly, most of them have been limited to the rhomboidal or hexagonal ones. The successful examples of triangular metallodendrimers have been relatively few, presumably due to the difficulty in finding the appropriate corner unit. Moreover, the triangular metallacycles sometimes suffer from the noticeable equilibrium with the molecular squares when the flexible building blocks are employed [26, 27, 28]. Thus, the construction of triangular metallodendrimers with well-defined shape and size is still a challenge [29].Moreover, the hierarchical self-assembly behaviors of triangular metallodendrimers have been relatively unexplored.
Herein, we successfully synthesized two discrete peripherally dimethyl isophthalate (DMIP) functionalized poly(benzyl ether) metallodendrimers featuring a well-defined triangular cavity at their cores via coordination-driven self-assembly (Scheme 1). Interestingly, it was found that the dendron generation played an important role in hierarchical self-assembly behaviors of the obtained triangular metallodendrimers. For example, the firstgeneration of metallodendrimer was able to hierarchically selfassemble into the spherical nanostructures in various mixed solvents. However, the nanofibers were observed for the secondgeneration of metallodendrimer under the similar conditions. Furthermore, the 1H NMR and fluorescence spectroscopy studies revealed that the formation of ordered nanostructures was mainly driven by intermolecular π-π stacking interaction imposed by the poly(benzyl ether) dendrons.
|
Download:
|
| Scheme 1.Synthetic routes of dendrimer ligands a and b. | |
Under an atmosphere of nitrogen, 5.0 mL of toluene and 2.0 mL of Et3N were added to a mixture of 4-ethynylpyridine hydrochloride (180.0 mg, 1.29 mmol), compound 7 (250.0 mg, 0.32 mmol), CuI (15 mg, 0.08 mmol), and Pd(PPh3)4 (75 mg, 0.06 mmol). The mixture was stirred at 95 ℃ for 27 h. Then the solvents were removed in vacuo, and the residue was purified by column chromatography on silica gel (dichloromethane/methanol = 20/1) to give a (93 mg, 35.1%) as a yellow solid. Rf = 0.6 (dichloromethane/ methanol = 20/1). M.p. 232 ℃. 1H NMR (CDCl3, 400 MHz): δ 8.93 (s, 2H), 8.64 (d, 4H,J = 6.0 Hz), 8.= (s, 2H), 8.29 (d, 2H,J = 8.4 Hz), 8.27 (s, 4H), 7.83 (d, 2H,J = 8.8 Hz), 7.50 (d, 4H,J = 6.0 Hz), 5.35 (s, 4H), 3.91 (s, 12H). 13C NMR (CDCl3, 100 MHz): δ 166.1, 150.3, 144.6, 138.6, 133.0, 131.4, 131.3, 130.5, 130.4, 129.8, 128.5, 127.3, 125.8, 123.2, 120.6, 94.2, 88.1, 75.0, 52.7. ESI-MS: 825.=(M + H+).
2.2. Synthesis of the ligand bFollowing the procedure for the preparation of a. Compound 8 (220.0 mg, 0.15mmol), 4-ethynylpyridine hydrochloride (110.0mg, 0.79mmol), CuI (15 mg, 0.08mmol), Pd(PPh3)4 (40 mg, 0.035mmol), toluene (3.0 mL) and Et3N (2.0 mL) yielded b (100.0 mg, 43.6%) as a yellow solid after purification by column chromatography (dichloromethane/methanol = 20/1). Rf = 0.4 (dichloromethane/methanol = 20/1). M.p. 180 ℃. 1H NMR (CDCl3, 400 MHz): δ 8.92 (s, 2H), 8.64 (d, 4H,J = 4.0 Hz), 8.33 (d, 2H,J = 8.0 Hz), 8.167 (s, 4H), 7.82 (d, 2H,J = 8.4 Hz), 7.73 (s, 8H), 7.60 (s, 4H), 7.57 (s, 2H), 7.50 (d, 4H,J = 4.4 Hz), 5.35 (s, 4H) 5.08 (s, 8H), 3.88 (s, 24H). 13C NMR (CDCl3, 100MHz): δ 166.1, 159.0, 150.3, 144.8, 138.7, 137.7, 132.3, 131.5, 130.5, 130.1, 128.5, 127.3, 127.1, 126.6, 125.9, 123.3, 120.1, 94.33, 88.0, 75.6, 70.3, 52.7, 30.1.MALDI-TOFMS of b: calcd. for C86H68N2O22 [M + 2H+]2+: 1482.43, found: 1482.36.
2.3. Synthesis of the triangular metallodendrimer AThe dipyridyl donor ligand a (10.04 mg, 12.2 mmol) and the organoplatinum 1808 acceptor 10 (12.88 mg, 12.2 mmol) were weighed accurately into a glass vial. To the vial was added 1.2 mL of acetone and 0.2 mL of H2O, and the reaction solution was then stirred at =˚ C for 15 h to yield a homogeneous orange solution. Then acetone (3.0 mL) was added, followed by the addition of a saturated aqueous solution of KPF6 into the bottle with continuous stirring (5 min) to precipitate the product (Scheme 2). The reaction mixture was centrifuged, washed three times with water, and dried. The pare-yellow product A (22.5 mg, 98%) was collected and redissolved in CD2Cl2 for NMR analysis. 1H NMR (CD2Cl2, 400 MHz): δ 9.15 (s, 6H), 8.70 (d, 12H,J = 5.6 Hz), 8.57 (s, 6H), 8.34 (d, 6H,J = 8.4 Hz), 8.29 (s, 12H), 7.88-7.92 (m, 18H), 7.09 (s, 12H), 5.38 (s, 12H), 3.93 (s, 36H), 1.38 (m, 72H), 1.20 (s, 108H). 31P NMR (CD2Cl2, 161.9 MHz): δ 12.95 (1JPt-P = 2716.7 Hz). CSI-TOFMS of A,[M-3PF6-]3+: 1908.53, found: 1908.62; [M-4PF6-]4+: 1395.16, found: 1395.20; [M-5PF6-]5+: 1087.13, found: 1087.19.

|
Download:
|
| Scheme 2.Self-assembly of dipyridyl donors a and b with the corresponding diplatinum(Ⅱ) acceptor 10 into supramolecular metallacycles A and B. | |
Following the procedure for the preparation of A. Compound b (15.09 mg, 10.19 mmol),10 (10.83 mg, 10.19 mmol), acetone (1.8 mL), water (0.3 mL) yielded B (25.6 mg, 99%) as a pare-yellow solid. 1H NMR (CD2Cl2, 400 MHz): δ 9.15 (s, 6H), 8.70 (d, 12H,J = 5.6 Hz), 8.40 (d, 6H,J = 8.4 Hz), 8.18 (s, 12H), 7.85-7.91 (m, 18H), 7.75 (s, 24H), 7.61 (s, 12H), 7.59 (s, 6H), 7.09 (s, 12H), 5.38 (s, 12H), 5.10 (s, 24H), 3.89 (s, 72H), 1.38 (m, 72H), 1.18 (s, 108H). 31P NMR (CD2Cl2, 161.9 MHz): δ 12.96 (1JPt-P = 2723.2 Hz). CSI-TOFMS of B,[M-4PF6-]4+: 1887.5, found: 1887.5; [M-5PF6-]5+: 1481.0, found: 1481.2.
3. Results and discussionThe newly designed 60° dendritic dipyridyl donors a and b were synthesized through a two-step sequence as shown in Schemes S3 in Supporting information. The DMIP-functionalized poly(benzyl ether) dendrons were introduced by an etherification reaction of compound 6 with the corresponding dendritic bromides. The subsequent Sonogashira coupling of 7 or 8 with 4-ethynylpyridine hydrochloride yielded 60° donor ligands a or B in the presence of Pd(PPh3)4 and CuI as catalysts.
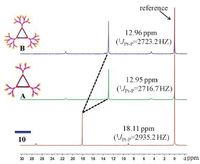
The metallacycles A and B were well characterized by multiple nuclear (1H and 31P) NMR spectroscopy, which revealed the formation of discrete and highly symmetric species. For example, the protons on the pyridine rings of donor B exhibited obvious downfield shifts (ca. Ha, 0.06 ppm; Hb, 0.41 ppm), which was caused by the loss of the electron density upon coordination of the pyridine-N atoms with the Pt(Ⅱ) metal center (Fig. 1). Moreover, each 31P{1H} NMR spectrum of the metallacycles A and B displayed a sharp singlet at 12.95 ppm for A and 12.96 ppm for B; these peaks were shifted upfield from that of the starting platinum acceptor 10 by -5.16 ppm (Fig. 2). This change, as well as the decrease in coupling of flanking 195Pt satellites (ca. ΔJ = -212.0 for A; ΔJ = -218.5 Hz for B), is consistent with the electron back-donation from the platinum atoms.

|
Download:
|
| Fig. 1.The partial 1H NMR spectra (400 MHz, in CD2Cl2, 298 K) of acceptor 10, dendritic dipyridine donor b, and their corresponding self-assembled metallacycle B. | |
|
Download:
|
| Fig. 2.The 31P NMR spectra (161.9 MHz, in CD2Cl2, 298 K) of acceptor 10, metallacycle A, and metallacycle B. | |
Furthermore, the structure of metallacycles A and B were confirmed by CSI-TOF-MS [30], which provided strong support for the formation of desired triangular metallodendrimers. For example, the CSI-TOF-MS spectrum of A displayed three peaks (Fig. S23 in Supporting information), corresponding to different charge states that were resulted from the loss of PF6- counterions,[M-3PF6-]3+,[M-4PF6-]4+, and [M-5PF6-]5+, where M represents the intact assembly. Further investigation revealed that each isotope pattern of these peaks was in good agreement with the corresponding simulated result. Similarly, in the case of metallacycle B, two peaks that agreed well with the corresponding simulated isotope patterns were found in the CSI-TOF-MS spectrum (Fig. S24 in Supporting information). All the aforementioned data support the highly efficient construction of dendrimers- functionalized triangular metallacycles with a well-defined shape and size through coordination-driven self-assembly, thus avoiding the time-consuming procedures and low yields often encountered in covalent synthesis protocols.
All attempts to grow X-ray-quality single crystals of triangular metallodendrimers A and B were unsuccessful. Thus, the PM6 semiempirical molecular orbital method was employed to optimize the molecular geometry of A and B. It was found that each metallodendrimer featured a roughly planar triangular ring at its core surrounded by the dendron subunits at corners (Fig. 3 and Fig. S5 in Supporting information). Moreover, the size of the triangular metallodendrimers was also determined. For instance, in the case of B, the triangular metallacycle had an internal diameter of 2.6 nm.

|
Download:
|
| Fig. 3.Geometric structure of B optimized by the PM6 semiempirical molecular orbital method. | |
Recently, DMIP-functionalized poly(benzyl ether) dendrons, which showed multiple intermolecular interactions (e.g.,π-π stacking and CH-π interactions), have been employed for the construction of organogel and the ordered nanostructures [31, 32, 33]. Inspired by these reports, the hierarchical self-assembly behaviors of triangular metallodendrimers were studied in various solvents by using scanning electron microscopy (SEM). Interestingly, it was found that both the metallacycles A and B were able to hierarchically self-assemble into the ordered nanostructures. As shown in Fig. 4, the first-generation metallodendrimer A exhibited spherical morphology with a relatively uniform distribution in various mixed solvents, such as dichloromethane and toluene (v/v, 9/1), dichloromethane and anisole (v/v, 10/1), acetone and ethanol (v/v, 10/1). In order to obtain the additional information about the nature of spherical morphology, transmission electron microscopy (TEM) measurements were performed. Interestingly, it was found that the spherical structures prepared in dichloromethane and toluene exhibited a clear contrast between the interior and periphery, which is the typical characteristic of vesicular structures (Fig. 5a). On the contrary, the spherical structures prepared in acetone and ethanol did not show such clear contrast between the interior and periphery, suggesting the existence of solid sphere (Fig. 5b). Notably, the well-defined spherical nanostructures have received increasing attention because of their various applications in the fields of catalysis, sensors, and drug delivery, etc. [34, 35, 36]. However, the second-generation metallodendrimer B, which has the same metallacyclic scaffold at the core with A, displayed the nanofibers morphology in the similar mixed solvents. These results indicated that the dendron generation has significant influences on the hierarchical self-assembly behaviors of triangular metallodendrimers.

|
Download:
|
| Fig. 4.Selected SEM images of the triangular metallodendrimers A (a-c) and B (d-e) in different solvents. (a) Dichloromethane and toluene (v/v, 9/1); (b) dichloromethane and anisole (v/v, 10/1); (c) acetone and ethanol (v/v, 10/1); (d) dichloromethane and toluene (v/v, 20/1); (e) dichloromethane and anisole (v/v, 3/1); (f) dichloromethane and tetrachloromethane (v/v, 3/1). | |

|
Download:
|
| Fig. 5.Selected TEM images of the triangular metallodendrimer A in different solvents. (a) Dichloromethane and toluene (v/v, 9/1); (b) acetone and ethanol (v/v, 10/1). | |
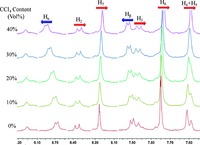
With the aim to investigate the driving forces for the formation of such ordered nanostructures, the 1H NMR study was performed with different solvent compositions. According to the previous reports [33], the tetrachloromethane was selected as the poor solvent because it could effectively induce the enhancement of other weak non-covalent interactions. As shown in Fig. 6, upon the addition of tetrachloromethane into the CD2Cl2 solution of B, the protons on the pyridine rings exhibited obvious downfield shifts. In contrast, the protons H2-H7 on the phenanthrene rings and benzene rings displayed a gradual upfield shifts, indicating the existence of intermolecular π-π stacking interaction between DMIP-functionalized poly(benzyl ether) dendrons [33]. In addition, the aggregation behavior of triangular metallodendrimer B was further studied by using fluorescence spectroscopy. Upon photoexcitation at 383 nm, the metallodendrimer B ([B] = 5 ± 10-5 mol/L) exhibited an intense emission band at 502 nm in a dilute solution of DCM. However, it was found that the fluorescence intensity of B gradually decreased and the emission maximum exhibited a slight blue-shift (16 nm) when the volume ratio of tetrachloromethane to DCM was increased (Fig. 7). With reference to previous spectroscopic studies on DMIP-functionalized poly(benzyl ether) dendrons complexes [33], these changes suggested that the intermolecular π-π stacking interactionwas enhanced upon increasing the fraction of poor solvent (CCl4), which was good agreement with the result obtained from 1H NMR study. Based on these experiment results, we proposed that the metallodendrimer B might feature stronger intermolecular π-π stacking interaction than that of the metallodendrimer A because of the higher dendron generation, thus resulting in the formation of intertwined three-dimensional networks of fibrillar structure.
|
Download:
|
| Fig. 6.The partial 1H NMR spectra of B ([B] = 7 × 10-4 mol/L) in the CD2Cl2-CCl4 mixed solvents with different CCl4 volume ratios at room temperature. | |
In summary, we have successfully prepared a new family of triangular metallodendrimers from the self-assembly of newly designed 60° dendritic dipyridyl donors a or b with the 1808 di- Pt(Ⅱ) acceptor 10 via coordination-driven self-assembly. Importantly, compared to some previous successful examples of supramolecular triangles, this study provides a highly efficient approach to the construction of such species without templates. Moreover, all the obtained triangular metallodendrimers were well characterized by multiple nuclear (1H and 31P) NMR spectroscopy, CSI-TOF-MS, and molecular simulation. The study of aggregation behaviors revealed that the metallacycles A and B were able to hierarchically self-assemble into the different nanostructures under the similar condition. Further investigation suggested that the different aggregation behaviors might be derived from the change in the strength of intermolecular π-π stacking interaction. This work not only represents one of few successful examples of triangular metallodendrimers with the well-defined shape and size, but also provides new insight into the design of the ordered nanostructures.
AcknowledgmentsL. Xu acknowledges the financial support of the National Natural Science Foundation of China (No. 21302058). H.-B. Yang acknowledges the financial support of the Key Basic Research Project of Shanghai Science and Technology Commission (No. 13JC1402200).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.03.017.
| [1] | J. Yang, Q. Zhang, H. Chang, Y. Cheng, Surface-engineered dendrimers in gene delivery, Chem. Rev. 115(2015) 5274-5300. |
| [2] | Y.M. Ye, Y. Feng, Q.H. Fan, Asymmetric hydrogenation in the core of dendrimers, Acc. Chem. Res. 47(2014) 2894-2906. |
| [3] | A.W. Bosman, H.M. Janssen, E.W. Meijer, About dendrimers:structure, physical properties, and applications, Chem. Rev. 99(1999) 1665-1688. |
| [4] | D. Astruc, E. Boisselier, C. Ornelas, Dendrimers designed for functions:from physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine, Chem. Rev. 110(2010) 1857-1959. |
| [5] | L. Xu, L.J. Chen, H.B. Yang, Recent progress in the construction of cavity-cored supramolecular metallodendrimers via coordination-driven self-assembly, Chem. Commun. 50(2014) 5156-5170. |
| [6] | C.M. Cardona, S. Mendoza, A.E. Kaifer, Electrochemistry of encapsulated redox centers, Chem. Soc. Rev. 29(2000) 37-42. |
| [7] | S. Serroni, S. Campagna, F. Puntoriero, C.D. Pietro, N.D. McClenaghan, F. Loiseau, Dendrimers based on ruthenium(Ⅱ) and osmium(Ⅱ) polypyridine complexes and the approach of using complexes as ligands and complexes as metals, Chem. Soc. Rev. 30(2001) 367-375. |
| [8] | D. Astruc, C. Ornelas, J. Ruiz, Metallocenyl dendrimers and their applications in molecular electronics, sensing, and catalysis, Acc. Chem. Res. 41(2008) 841-856. |
| [9] | R. Chakrabarty, P.S. Mukherjee, P.J. Stang, Supramolecular coordination:selfassembly of finite two- and three-dimensional ensembles, Chem. Rev. 111(2011) 6810-6918. |
| [10] | Y.F. Han, W.G. Jia, W.B. Yu, G.X. Jin, Stepwise formation of organometallic macrocycles, prisms and boxes from Ir, Rh and Ru-based half-sandwich units, Chem. Soc. Rev. 38(2009) 3419-3434. |
| [11] | L. Xu, Y.X. Wang, L.J. Chen, H.B. Yang, Construction of multiferrocenyl metallacycles and metallacages via coordination-driven self-assembly:from structure to functions, Chem. Soc. Rev. 44(2015) 2148-2167. |
| [12] | B. Olenyuk, J.A. Whiteford, A. Fechtenkö tter, P.J. Stang, Self-assembly of nanoscale cuboctahedra by coordination chemistry, Nature 398(1999) 796-799. |
| [13] | Q.F. Sun, J. Iwasa, D. Ogawa, et al., Self-assembled M24L48 polyhedra and their sharp structural switch upon subtle ligand variation, Science 328(2010) 1144-1147. |
| [14] | P. Mal, B. Breiner, K. Rissanen, J.R. Nitschke, White phosphorus is air-stable within a self-assembled tetrahedral capsule, Science 324(2009) 1697-1699. |
| [15] | D.M. Kaphan, M.D. Levin, R.G. Bergman, K.N. Raymond, F.D. Toste, A supramolecular microenvironment strategy for transition metal catalysis, Science 350(2015) 1235-1238. |
| [16] | Y.Y. Zhang, X.Y. Shen, L.H. Weng, G.X. Jin, Octadecanuclear macrocycles and nonanuclear bowl-shaped structures based on two analogous pyridyl-substituted imidazole-4,5-dicarboxylate ligands, J. Am. Chem. Soc. 136(2014) 15521-15524. |
| [17] | D. Samanta, P.S. Mukherjee, Sunlight-induced covalent marriage of two triply interlocked Pd6 cages and their facile thermal separation, J. Am. Chem. Soc. 136(2014) 17006-17009. |
| [18] | S. Lö ffler, J. Lübben, L. Krause, et al., Triggered exchange of anionic for neutral guests inside a cationic coordination cage, J. Am. Chem. Soc. 137(2015) 1060-1063. |
| [19] | N. Kishi, M. Akita, M. Kamiya, S. Hayashi, H.F. Hsu, M. Yoshizawa, Facile catch and release of fullerenes using a photoresponsive molecular tube, J. Am. Chem. Soc. 135(2013) 12976-12979. |
| [20] | Z.Y. Li, Y. Zhang, C.W. Zhang, et al., Cross-linked supramolecular polymer gels constructed from discrete multi-pillar[5] arene metallacycles and their multiple stimuli-responsive behavior, J. Am. Chem. Soc. 136(2014) 8577-8589. |
| [21] | B. Jiang, J. Zhang, J.Q. Ma, et al., Vapochromic behavior of a chair-shaped supramolecular metallacycle with ultra-stability, J. Am. Chem. Soc. 138(2016) 738-741. |
| [22] | L.J. Chen, Y.Y. Ren, N.W. Wu, et al., Hierarchical self-assembly of discrete organoplatinum(Ⅱ) metallacycles with polysaccharide via electrostatic interactions and their application for heparin detection, J. Am. Chem. Soc. 137(2015) 11725-11735. |
| [23] | X.Q. Wang, W. Wang, G.Q. Yin, et al., Cross-linked supramolecular polymer metallogels constructed via a self-sorting strategy and their multiple stimulusresponse behaviors, Chem. Commun. 51(2015) 16813-16816. |
| [24] | N.W. Wu, L.J. Chen, C. Wang, et al., Hierarchical self-assembly of a discrete hexagonal metallacycle into the ordered nanofibers and stimuli-responsive supramolecular gels, Chem. Commun. 50(2014) 4231-4233. |
| [25] | J. Zhang, R. Marega, L.J. Chen, et al., Hierarchical self-assembly of supramolecular hydrophobic metallacycles into ordered nanostructures, Chem. Asian J. 9(2014) 2928-2936. |
| [26] | M. Schweiger, S.R. Seidel, A.M. Arif, P.J. Stang, Solution and solid state studies of a triangle-square equilibrium:anion-induced selective crystallization in supramolecular self-assembly, Inorg. Chem. 41(2002) 2556-2559. |
| [27] | F.A. Cotton, C. Lin, C.A. Murillo, A neutral triangular supramolecule formed by Mo24+ units, Inorg. Chem. 40(2001) 575-577. |
| [28] | M. Fujita, O. Sasaki, T. Mitsuhashi, et al., On the structure of transition-metallinked molecular squares, Chem. Commun. (1996) 1535-1536. |
| [29] | Q. Han, L.L. Wang, Q.J. Li, et al., Synthesis of triangular metallodendrimers via coordination-driven self-assembly, J. Org. Chem. 77(2012) 3426-3432. |
| [30] | J. He, Z. Abliz, R. Zhang, Y. Liang, K. Ding, Direct on-line method to monitor the dynamic structure of noncovalent titanium complexes in solution by using cold-spray ionization time-of-flightmass spectrometry, Anal. Chem. 78(2006) 4737-4740. |
| [31] | L.J. Chen, G.Z. Zhao, B. Jiang, et al., Smart stimuli-responsive spherical nanostructures constructed from supramolecular metallodendrimers via hierarchical self-assembly, J. Am. Chem. Soc. 136(2014) 5993-6001. |
| [32] | G.Z. Zhao, L.J. Chen, W. Wang, et al., Stimuli-responsive supramolecular gels through hierarchical self-assembly of discrete rhomboidal metallacycles, Chem. Eur. J. 19(2013) 10094-10100. |
| [33] | Y. Feng, Z.T. Liu, J. Liu, et al., Peripherally dimethyl isophthalate-functionalized poly(benzyl ether) dendrons:a new kind of unprecedented highly efficient organogelators, J. Am. Chem. Soc. 131(2009) 7950-7951. |
| [34] | Y. Lu, J. Liu, Smart nanomaterials inspired by biology:dynamic assembly of errorfree nanomaterials in response to multiple chemical and biological stimuli, Acc. Chem. Res. 43(2007) 315-323. |
| [35] | B. Rybtchinski, Adaptive supramolecular nanomaterials based on strong noncovalent interactions, ACS Nano 5(2011) 6791-6818. |
| [36] | K.K. Cotí, M.E. Belowich, M. Liong, et al., Mechanised nanoparticles for drug delivery, Nanoscale 1(2009) 16-39. |
 2016, Vol.27
2016, Vol.27 


