b Key Laboratory of Organo-pharmaceutical Chemistry, Gannan Normal University, Ganzhou 341000, China
Coordination chemistry has emerged as a promising strategy for the construction of advanced materials with well-defined nanoscale structures [1, 2, 3, 4, 5]. The non-covalent interactions between organic ligands and metals are an essential feature in the design of materials’ structure. In particular, directional interactions between the designed functional groups control the supramolecular structure. Metal-organic complexes have promising prospects for the applications in the fields of chemical separation, catalysis, optoelectronics, magnetic, and are a class of important source materials in preparing nano-devices [6, 7, 8, 9, 10]. Although the research is mainly focused on their three-dimensional crystals at present, this class of complexes are also a hot research topic in supramolecular chemistry [4, 5, 6, 7, 8, 9, 10].
Scientists have evaluated the assembly structures and properties of such aggregates on surface through the scanning tunneling microscopy (STM) technique, which can provide clear images with atomic resolution [12, 13, 14, 15, 16, 17]. Hereinto, metal complexes of porphy-rin and phthalocyanine have received considerable attention due to their large π systems. Based on the self-assemblies of porphyrins and phthalocyanines as well as the coadsorption structure of their mixtures [18, 19], various stable and periodic structures have been prepared using intermolecular coordination interactions, such as one-dimensional coordination polymer, two-dimensional coordination grid and three-dimensional complex structures [4, 11, 17, 20, 21]. The other reason of such studies is that metal- ligand bonds carry great advantages such as fewer synthesis steps, faster synthesis process, self-repair ability, which are effective in self-assembling and preparing periodic nanostructures.
Recently, we studied the adsorption behavior of coordination complexes in a supramolecular template on a highly oriented pyrolytic graphite (HOPG) surface using STM under atmospheric conditions [22, 23]. It was found that two zinc phthalocyanines (ZnPc) can coordinate with 1, 3-di(4-pyridyl)propane (DPP) and formed a well-ordered self-assembled structure with a molecular template. From both fundamental and technological points of view, formation and characterization of axial coordination with the central metal are of great importance because the obtained complexes are closely related to the crystal engineering applications. While previous reports on coordinated cluster ZnPc-DPP- ZnPc are encouraging for surface aggregation in different templates, rational design of materials is in question. Is there only ZnPc suitable for forming such complexes? How to design the chemical structures?
Therefore, this study will be focused on other molecules that can form coordinated complex structures on the HOPG surface. On the basis of previous study, in this work we firstly studied the physical and chemical co-absorption behaviors of ZnPc and zinc tetraphenylporphyrin (ZnTPP) with DPP. And then we selected another analogous dipyridyl molecule 1, 2-di(4-pyridyl)ethylene (DPE) to investigate whether it can coordinate with ZnPc on the HOPG surface. After analysis of the results, it can be concluded that new complexes with different structures were fabricated on the HOPG surface and a synergistic effect of a pre-fabricated template was also observed.
2. ExperimentalMaterials: The materials studied include 1, 3, 5-tris(10-carboxydecyloxy) benzene (TCDB), Zinc phthalocyanine (ZnPc), zinc tetraphenylporphyrin (ZnTPP), 1, 3-di(4-pyridyl)propane (DPP), 1, 2-di(4-pyridyl)ethylene(DPE).TCDB was synthesized according to the methods reported. ZnPc, ZnTPP, DPP, DPE, and solvent 1- phenyloctane were purchased from Tokyo Chemical Industry Co., Ltd. These reagents were used without any purification.
Preparation of sample and measurements: The compounds (TCDB, ZnPc, ZnTPP, DPP, DPE) were dissolved in 1-phenyloctane with concentrations less than 10-4 mol/L. The solutions of the target compoundswere deposited onto the freshly cleaved highly oriented pyrolytic graphite (HOPG) surface. STM imaging was performed at the solid-liquid interface after a short period of time under ambient conditions. Then, 0.4 mL of 1-phenyloctane solution containing DPP or DPE was deposited onto the assembly structure. STM was conducted at the solid-liquid interface after staying for 20 min. STM with a digital instruments nanoscope Ⅲ controller was used to acquire the reported constant current images using Pt0.8Ir0.2 tips.
3. Results and discussion 3.1. Self-assembly of ZnPc/ZnTPP in TCDB templateThe chemical structures of studied compounds are shown in Scheme 1. Because of the relative inertness on the HOPG surface, ZnPc and ZnTPP molecules diffuse over the surface, and thus single isolated molecules cannot be seen by the STM technique udner ambient conditions. Very recently, porphyrin and phthalocyanine molecules have been investigated on several metal surfaces [23, 24, 25, 26, 27, 28, 29]. In this study, a network of 1, 3, 5-tris(10-carboxydecyloxy) benzene (TCDB) was formed and used as a template for the adsorption of ZnPc and ZnTPP molecules on the HOPG surface [30, 31], and the dimeric complexes of ZnPc or ZnTPP bridged by the DPP or DPE ligand formed in a TCDB template on surface were investigated.
|
Download:
|
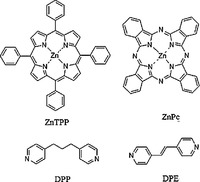
| Scheme 1.Chemical structures of ZnPc, ZnTPP, DPP and DPE. | |
Firstly, the formation of two-dimensional bimolecular ZnPc and ZnTPP structures in TCDB is observed by STM. When ZnPc and ZnTPP are deposited onto the HOPG surface in a 1:1 molar ratio, a well-ordered closely-packed bimolecular structure of ZnPc and ZnTPP can be observed as shown in Fig. 1a. It should be noted that ZnTPP molecules cannot be accommodated by the TCDB cavities possible due to the molecular size matching and the weak guesthost intermolecular interactions, while one TCDB cavity can hold one or two ZnPc molecules [23]. From the point of view of the chemical structure, ZnTPP exhibits relatively high-degree distortion, while ZnPc has a robust and planar aromatic structure that contributes to the aggregation on surface. As marked by the white rectangle, there might still be a small region in which one TCDB cavity holds two ZnPc molecules. In other area, however, ZnTPP and ZnPc coadsorbed in one TCDB cavity, and formed the hostguest structure.

|
Download:
|
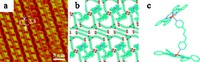
| Fig. 1.(a) Large-scale and (b) high-resolution STM images for the self-assembled structure of ZnTPP and ZnPc in a TCDB network. (c) The suggested molecularmodel of image (a) and (b). | |
A high-resolution STM image displayed in Fig. 1b shows a looser molecular packing than the ZnPc case. It is proposed that a wellordered 1:1 structure is shown with one ZnPc and one ZnTPP held in each TCDB cavity. After careful analysis, we can find that the molecular packing positions are slightly different between strips marked by the red and light blue arrows, respectively. Molecules ZnTPP and ZnPc were represented by red and light blue squares. In the region highlighted by white dotted rectangle, the relative position of ZnTPP and ZnPc in these two strips changed. From the proposed schematic molecular model shown in Fig. 1c, ZnTPP and ZnPc in one TCDB cavity might move and formed such structures. It should be noticed that there is no remarkable contrast in tunneling current between ZnPc and ZnTPP, therefore, it is difficult to clearly label molecules at molecular level based on the chemical identification. So, here one molecule was assigned to ZnPc and the other one was assigned to ZnTPP molecule.
Why ZnPc and ZnTPP can produce such an ordered twodimensional structure? The intermolecular interactions between ZnPc and ZnTPP as well as with TCDB might play a significant role. Usually, in the host-guest composite system including multicomponents, there are not only intermolecular hydrogen bonds and van der Waals force between host and guest but also significant interactions between guests [32, 33, 34, 35]. The synergetic effect of hostguest and guest-guest interactions results in both selective and competitive absorption of guests in the host template. Consequently, awell-ordered bimolecular self-assembled structure of ZnPc and ZnTPP through the effective intermolecular interactions formed.
3.2. Formation of ZnPc-DPP-ZnTPP in TCDB templateAnd then ligand DPP was added onto the ZnPc/ZnTPPmonolayer, after a short period of time, STM experiments were performed, revealing a new structure was obtained as shown in Fig. 2a. The length (L) of the bright rectangle is measured to be 3.1 nm, which is approximately the same size as the summarized length of one ZnPc molecule (1.2 × 1.2 nm), one ZnTPP (1.0 × 1.0 nm) and one DPP molecule (1.2 nm), which indicates that ZnPc-DPP-ZnTPP complexes formed after the addition of DPP [23, 35, 36]. ZnTPP is a nonplanarmolecule, and hence its assembly structure in the TCDB grid is not stable. In other words, ZnTPP molecules cannot be stably adsorbed in the TCDB cavities. When ZnTPP and ZnPc were mixed and co-assembled in one TCDB grid, four edged benzene rings of ZnTPPwillbe distortedowing toweak interactionwith the substrate. The distortion could cause both ZnPc and ZnTPPmove into a defined space. As a result, ZnPc and ZnTPP in the template can move and coordinate withDPP after the addition of DPP. Consequently, a novel heterocaryotic ∀-shaped complex was produced on the surface. Additionally, the conformationof the ∀-shapedcomplex onsurface is shown in Fig. 2c, displaying a suggested molecular model. This ∀- shaped complex adopts a similar v conformation on surface possible due to the v-shaped structure of DPP [23]. It indicates that the interactions of ZnPc and ZnTPP with substrateHOPGareweaker than the coordination interactions ofDPP withZnPc and ZnTPP.Moreover, it needs to be pointed out that it is difficult to obtain the heterocaryotic bridged-dimeric supramolecular complexes by traditional crystal engineering methods. Therefore, it is easy to believe that intermolecular interactions between the TCDB template and absorbers ZnPc and ZnTPP are the main reason of the occurrence of such abnormal coordination.
|
Download:
|
| Fig. 2.(a) STM image for the self-assembled structure of ZnTPP and ZnPc in TCDB networks after addition of DPP molecule. (b) The proposed molecular model for the observed structure in (a). (c) The suggested conformation of individual ZnPc–DPP–ZnTPP complex. | |
As mentioned above, it was concluded that one ZnPc and one ZnTPP molecule were immobilized into one TCDB cavity although there is no evidence to prove which is which. In our previous study of coordination between ZnPc and DPP, we found that DPP coordinated with ZnPc when only one ZnPc molecule was immobilized into the TCDB cavity [22]. However, if there are two ZnPc molecules in each TCDB cavity, the coordination cannot happen because ZnPc molecules cannot move freely in the cavity due to the steric effect of the cavity. Additionally, one TCDB cavity cannot accommodate two ZnTPP molecules. Therefore, we can conclude that after adding DPP onto the monolayer of the ZnPc and ZnTPP mixture with 1:1 molar ratio deposited onto the TCDB template, new coordination might have occurred.
A subsequent question is whether any other ligands can connect two ZnPc molecules. And then another dipyridyl ligand was selected and the chemical structure is shown in Scheme 1. It is interesting to notice that a new phenomenon happened after DPE replaces DPP. A new TCDB/ZnPc binary structure, in which one TCDB cavity can accommodate one ZnPc molecule, was formed after the DPE ligand was added. After a sufficiently long time, two kinds of domains A and B with ordered structure appeared, as shown in Fig. 3b. Meanwhile, these two coexisting phases were imaged by STM with higher resolution. In the high-resolution STM image, the molecular width (WA) in the A region can be measured to be circa 2.7 nm, and the height of molecule is measured to be circa 0.4 nm. In the B region, the width (WB) and the height of the molecule are measured to be 3.1 nm and 0.1 nm, respectively. Therefore, we considered that in the regions A and B, two ZnPc molecules coordinated with one DPE ligand and formed H-shaped complexes adsorbed in the TCDB grid. This structure is the same as the crystal structure formed by the common coordination method obtained previously in solutions [37].

|
Download:
|
| Fig. 3.(a) STM image for the self-assembled structure of ZnPc in TCDB networks with 1:1 molar ratio. (b) STM image for the self-assembled structure of ZnPc in TCDB networks after addition of DPE molecules. (c) and (d) High-resolution STM image of A and B shown in (b), respectively. (e) and (f) The illustrated molecular model in region A and B, respectively. (g) The molecular conformation of t ZnPc–DPE–ZnPc complexes. | |
Moreover, attention should be paid to the assembly structures of these two coordinated complexes, which are different in the region A and B as shown in Fig. 3e and f. In the A region, H-shaped complexes ZnPc-DPE-ZnPc almost vertically arrange on the HOPG substrate, while the other H-shaped complexes ZnPc-DPE-ZnPc assemble nearly parallelly to the substrate in the B region. Why the structure of ZnPc-DPE-ZnPc is different from that of ZnPc-DPP- ZnPc? The differences in the chemical structures between DPP and DPE probably induced differences in coordination.
Up to now, we have thoroughly investigated the supramolecular coordination behaviors occurring in the TCDB template by the STM technique. Through these supramolecular coordination caused by the collaborative effect, we obtained ZnPc-DPP-ZnPc, ZnPc-DPP-ZnTPP, and ZnPc-DPE-ZnPc complexes with special shapes and conformations on surface. A general procedure of fabricating heterocaryotic and homonuclear bridged-dimeric complexes on surface was identified. In the case of two molecules in a space, coordination can occur when these two molecules can rotate or the space can change to a sufficient degree. In the other case of one molecule in a space, coordination can occur when the space can change to the suitable size. These results indicate that the interactions between adsorbate and substrate are weak enough to ensure the lateral coordination interactions to play a featured role in the reassembly. This work also demonstrates that threedimensional arrays can be generated based on an initially desired monolayer template on surface. In these work, the supramolecular grid shows the ability of nano-reactor. Therefore, the coordination chemistry happening in the nano-reactor provides a newmethod to generate the coordination structures. It also exhibits a significant advantage in the preparation of new two-dimensional supramolecular aggregatedmaterials and crystal molecules. It can be predicted that further research and development in this field will greatly promote the synthesis and preparation of this type of materials.
4. ConclusionIn conclusion, we have observed heterocaryotic and homonuclear dimeric complexes of ZnTPP-ZnPc and ZnPc-ZnPc bridged by axial ligands DPP and DPEin a template of TCDB on the HOPG surface. These axial ligands-linked ZnTPP and ZnPcmolecules were effective to stabilize the coordination structures on the HOPG surface. This work succeeded in applying the concepts of crystal engineering to two-dimensional molecular systems by physisorption on surface. This will allow us to understand the stereo structure of coordinated aggregation and might be applicable to a variety of coordination assemblies.
Acknowledgments
Thisworkwas supported by theNational Basic Research Program of China (No. 2013CB934200), National Basic Research Program of China (No. 2012CB933001), The Chinese Academy of Sciences (No. YZ201318) and the National Natural Science Foundation of China (Nos. 21472029, 51173031, 91127043, 51203030, 51463002).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.01.033.
| [1] | S.S. Li, B.H. Northrop, Q.H. Yuan, L.J. Wan, P.J. Stang, Surface confined metallosupramolecular architectures:formation and scanning tunneling microscopy characterization, Acc. Chem. Res. 42(2009) 249-259. |
| [2] | L.J. Wan, Fabricating and controlling molecular self-organization at solid surfaces:studies by scanning tunneling microscopy, Acc. Chem. Res. 39(2006) 334-342. |
| [3] | H.L. Liang, Y. He, Y.C. Ye, et al., Two-dimensional molecular porous networks constructed by surface assembling, Coord. Chem. Rev. 253(2009) 2959-2979. |
| [4] | S. Mohnani, D. Bonifazi, Supramolecular architectures of porphyrins on surfaces:the structural evolution from 1D to 2D to 3D to devices, Coord. Chem. Rev. 254(2010) 2342-2362. |
| [5] | N. Li, X. Zhang, G.C. Gu, et al., Sierpiński-triangle fractal crystals with the C3v point group, Chin. Chem. Lett. 26(2015) 1198-1202. |
| [6] | J.S. Seo, D. Whang, H. Lee, et al., A homochiral metal-organic porous material for enantioselective separation and catalysis, Nature 404(2000) 982-986. |
| [7] | M. Eddaoudi, D.B. Moler, H.L. Li, et al., Modular chemistry:secondary building units as a basis for the design of highly porous and robust metal-organic carboxylate frameworks, Acc. Chem. Res. 34(2001) 319-330. |
| [8] | S. Kitagawa, R. Kitaura, S.I. Noro, Functional porous coordination polymers, Angew. Chem. Int. Ed. 43(2004) 2334-2375. |
| [9] | C. Sanchez, B. Julián, P. Belleville, M. Popall, Applications of hybrid organicinorganic nanocomposites, J. Mater. Chem. 15(2005) 3559-3592. |
| [10] | J.Y. Lee, O.K. Farha, J. Roberts, et al., Metal-organic framework materials as catalysts, Chem. Soc. Rev. 38(2009) 1450-1459. |
| [11] | G. Bottari, G. de la Torre, T. Torres, Phthalocyanine-nanocarbon ensembles:from discrete molecular and supramolecular systems to hybrid nanomaterials, Acc. Chem. Res. 48(2015) 900-910. |
| [12] | D. Heim, D. Écija, K. Seufert, et al., Self-assembly of flexible one-dimensional coordination polymers on metal surfaces, J.Am. Chem. Soc. 132(2010) 6783-6790. |
| [13] | H. Walch, J. Dienstmaier, G. Eder, et al., Extended two-dimensional metal-organic frameworks based on thiolate-copper coordination bonds, J. Am. Chem. Soc. 133(2011) 7909-7915. |
| [14] | S. Bernhard, K. Takada, D.J. Díaz, H.D. Abruñ a, H. Mürner, Enantiomerically pure chiral coordination polymers:synthesis, spectroscopy, and electrochemistry in solution and on surfaces, J. Am. Chem. Soc. 123(2001) 10265-10271. |
| [15] | J.I. Urgel, D. Ecija, W. Auwärter, J.V. Barth, Controlled manipulation of gadoliniumcoordinated supramolecules by low-temperature scanning tunneling microscopy, Nano Lett. 14(2014) 1369-1373. |
| [16] | T. Lin, G.W. Kuang, W.H. Wang, N. Lin, Two-dimensional lattice of out-of-plane dinuclear iron centers exhibiting kondo resonance, ACS Nano 8(2014) 8310-8316. |
| [17] | O. Shoji, H. Tanaka, T. Kawai, Y. Kobuke, Single molecule visualization of coordination-assembled porphyrin macrocycles reinforced with covalent linkings, J. Am. Chem. Soc. 127(2005) 8598-8599. |
| [18] | L. Scudiero, K.W. Hipps, D.E. Barlow, A self-organized two-dimensional bimolecular structure, J. Phys. Chem. B 107(2003) 2903-2909. |
| [19] | K. Suto, S. Yoshimoto, K. Itaya, Two-dimensional self-organization of phthalocyanine and porphyrin:dependence on the crystallographic orientation of Au, J. Am. Chem. Soc. 125(2003) 14976-14977. |
| [20] | P.C. van Gerven, J.A. Elemans, J.W. Gerritsen, et al., Dynamic combinatorial olefin metathesis:templated synthesis of porphyrin boxes, Chem. Commun. 28(2005) 3535-3537. |
| [21] | Q. Ferreira, L. Alcácer, J. Morgado, Stepwise preparation and characterization of molecular wires made of zinc octaethylporphyrin complexes bridged by 4, 4'-bipyridine on HOPG, Nanotechnology 22(2011) 435604. |
| [22] | J. Xu, Q.D. Zeng, Two-dimensional (2D) supramolecular coordination at liquid/solid interfaces studied by scanning tunneling microscopy, Chin. J. Chem. 33(2015) 53-58. |
| [23] | X.M. Zhang, Y.T. Shen, S. Wang, et al., One plus two:supramolecular coordination in a nano-reactor on surface, Sci. Rep. 2(2012) 742. |
| [24] | M. Koudia, M. Abel, C. Maurel, et al., Influence of chlorine substitution on the selfassembly of zinc phthalocyanine, J. Phys. Chem. B 110(2006) 10058-10062. |
| [25] | K. Nilson, P. Palmgren, J. Åhlund, et al., STM and XPS characterization of zinc phthalocyanine on InSb (001), Surf. Sci. 602(2008) 452-459. |
| [26] | S. Yoshimoto, Y. Honda, O. Ito, K. Itaya, Supramolecular pattern of fullerene on 2D bimolecular "chessboard" consisting of bottom-up assembly of porphyrin and phthalocyanine molecules, J. Am. Chem. Soc. 130(2008) 1085-1092. |
| [27] | P. Amsalem, L. Giovanelli, J.M. Themlin, T. Angot, Electronic and vibrational properties at the ZnPc/Ag (110) interface, Phys. Rev. B:Condens.Matter 79(2009) 235426. |
| [28] | Y.B. Li, K. Deng, X.K. Wu, et al., Molecular arrays formed in anisotropically rearranged supramolecular network with molecular substitutional asymmetry, J. Mater. Chem. 20(2010) 9100-9103. |
| [29] | S.R. Wagner, P.P. Zhang, Formation of highly ordered organic molecular thin films on deactivated si surfaces studied by scanning tunneling microscopy and low energy electron diffraction, J. Phys. Chem. C 118(2014) 2194-2201. |
| [30] | Y.T. Shen, L.J. Zeng, D. Lei, et al., Competitive adsorption and dynamics of guest molecules in 2D molecular sieves, J. Mater. Chem. 21(2011) 8787-8791. |
| [31] | Y.T. Shen, K. Deng, X.M. Zhang, et al., Selective and competitive adsorptions of guest molecules in phase-separated networks, J. Phys. Chem. C 115(2011) 19696-19701. |
| [32] | D.X. Wu, K. Deng, Q.D. Zeng, C. Wang, Selective effect of guest molecule length and hydrogen bonding on the supramolecular host structure, J. Phys. Chem. B 109(2005) 22296-22300. |
| [33] | X.M. Zhang, Q.D. Zeng, C. Wang, Host-guest supramolecular chemistry at solid-liquid interface:an important strategy for preparing two-dimensional functional nanostructures, Sci. China Chem. 57(2014) 13-25. |
| [34] | J. Xu, Q.D. Zeng, Construction of two-dimensional (2D) H-bonded supramolecular nanostructures studied by STM, Chin. Chem. Lett. 24(2013) 177-182. |
| [35] | W. Auwärter, A. Weber-Bargioni, A. Riemann, et al., Self-assembly and conformation of tetrapyridyl-porphyrin molecules on Ag (111), J. Chem. Phys. 124(2006) 194708. |
| [36] | X.H. Kong, Y.L. Yang, S.B. Lei, C. Wang, On the topography multiplicity of nonplanar titanyl (IV) phthalocyanine molecules and the STM imaging mechanism, Surf. Sci. 602(2008) 684-692. |
| [37] | Q.D. Zeng, D.X. Wu, C. Wang, et al., Bipyridine conformations control the solidstate supramolecular chemistry of Zinc (II) phthalocyanine with bipyridines, CrystEngComm 7(2005) 243-248. |
 2016, Vol.27
2016, Vol.27 


