b Center for Functional Nanomaterials, Brookhaven National Laboratory, Upton, Brookhaven, NY 11973, USA;
c School of Materials Science and Engineering, Tianjin University, Tianjin 300072, China
The massive consumption of global energy resources resulted in the shortage of fossil fuels and severe environmental problems. Proton exchange membrane fuel cells (PEMFCs) have attracted widespread interests due to their high energy density and negligible emission of harmful byproducts. Oxygen reduction reaction, the reaction rate control step in PEMFCs, has been extensively studied due to the urgent requirement of efficient electrocatalysts to accelerate its reaction rate. Platinum-based catalysts are well-known as the most active catalysts for the ORR. However, the scarcity resource, high cost and poor durability hinder its further commercialization. Therefore, it is of vital importance to explore platinum-free catalyst to improve the sluggish kinetics of the ORR [1].
In recent years, graphene-based nanomaterials have shown excellent electrocatalytic performance for ORR [2, 3]. However, the two-dimensional graphene sheets incline to agglomerate during thermal annealing and electrochemical measurements due to the strong π-π interaction. The agglomeration of graphene layers would significantly reduce the specific surface area and even lower the electrocatalytic performance. Therefore, it is of vital importance to explore proper methods to avoid the re-stacking of graphene layers. Yu et al. incorporated multi-walled carbon nanotubes (MWCNTs) with graphene layers to form a three dimensional N doped aerogel structure [4]. Qiao et al. grew graphene layers on hierarchical ordered porous carbon materials which combined the advantages of both hierarchical porous carbon and graphene [5]. Moreover, the kinetics of the ORR would be significantly enhanced via heteroatom (e.g. B [6, 7], N [8, 9, 10, 11], P [12, 13], S [14, 15] and I [16]) doping. Recent studies have also revealed the existence of a synergistic coupling effect, predicting that co-doping would offer much more active sites than single atomic doping [17].
In this paper, graphene nanoribbons/CNTs composite was successfully obtained by unzipping MWCNTs with different exfoliation degree. The CNT with partially unzipped outer-wall formed a remained inner CNT skeleton supported graphene nanoribbons, with a three dimensional structure, inhibiting the restacking of graphene layers. After heat-treatment with ammonium dihydrogen phosphate at high temperature, N and P atoms were successfully co-doped in the nanocomposite. The unique 3-D structure reserves both the properties of CNT and graphene, exhibiting excellent catalytic performance for the ORR with excellent onset and half-wave potential.
2. Experimental 2.1. Preparation of graphene/nanotubes compositeThe graphene nanoribbons/CNTs composite was prepared via a Hummers method [18]. First, 2.0 g of MWCNT (20-30 nm in diameter and 10-20 mmin length) was added into a 500 mL threenecked round-bottomed flask filled with 300 mL concentrated H2SO4. The mixture was continuously mechanical stirred for 12 h at room temperature. 4.0 g of KMnO4 was then slowly added into the flask and mechanical stirred for 1 h, and then heated to 70 ℃ for 1 h. After cooled down to room temperature, the mixture was poured into a 3000 mL baker filled with 25 mL of H2O2 and 500 mL of ice. Resulting precipitate was obtained by suction-filtered in air and washed with copious amounts of distilled water until the pH was 7 and then underwent a freeze-drying process. The obtained material was denoted as CNT-2 (the mass ratio of MWCNT to KMnO4 was 1:2). Similarly, by changing the ratio of MWCNT to KMnO4 to 1:0 and 1:4, the materials were denoted as CNT-0 and CNT-4, respectively.
2.2. Preparation of N/P co-doped exfoliation MWCNTThe mixture of 0.1 mg of partially unzipped CNTs and 1.0 g of ammonium dihydrogen phosphate (NH4H2PO4) was first grounded into fine powder in a mortar, and then annealed in a tube furnace at a heating rate of 10 ℃ min-1 for 2 h under the protection of N2 to obtain nitrogen and phosphorus co-doped graphene nanoribbons/ CNTs composite (NPCNT-2). For comparison, NSCNT-0 and NSCNT- 4 were also prepared accordingly.
2.3. Physical characterizationX-ray photoelectron spectroscopy (XPS) data were obtained by using an AXIS-ULTRA DLD-600W Instrument. Fourier transform infrared (FT-IR) spectra of the samples were collected on VERTEX 70, BRUKER Inc. S/TEM images were obtained using an 300 keV field-emission scanning transmission electron microscopy. Raman spectra were taken by a LabRam HR800 spectrometer with a 532 nm laser excitation.
2.4. Electrochemical measurementsThe electrochemical measurements were performed on a CHI 760e electrochemical workstation equipped with a high speed rotator from Pine Instruments in which a three-electrode system consisting of a glassy carbon working electrode (5 mm in diameter), a piece of carbon paper as counter electrode to eliminate the contamination from Pt, and reverse hydrogen electrode (RHE, Eθ = -0.768 V vs. SHE) as reference electrode at room temperature (298 K) in 0.1 mol L-1 KOH solution. 5 mg of samples was dispersed in 1 mL of isopropanol/Nafion hybrid solutions and ultrasonic dispersed to form homogeneous ink. 16 mL of ink was drop onto the glassy carbon substrate, and dried naturally. The mass loading of commercial Pt/C is about 15 mg cm-2 which worked as a benchmark for the comparison of the as synthesized samples. The cyclic voltammetry and linear sweep voltammetry of the samples were operated in N2- or O2- saturated 0.1 mol L-1 KOH solution at a scanning rate of 50 mV s-1 or sweep rate of 5 mV s-1. Electrons transfer number (n) was calculated from the following Koutecky-Levich (K-L) equation:


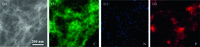
Graphene nanoribbons/CNTs composite derived from the oxidization and exfoliation of MWCNTs are usually employed as precursor for heteroatom doping. Fig. 1a and b shows the transmission electron microscopy (TEM) image of NPCNT-0 and dark-field scanning transmission electron microscopy (DF-STEM) image of NPCNT-4, respectively. NPCNT-0 remains integrated onedimensional tubular structure while NPCNT-4 was almost completely exfoliated and increased the layer thickness due to the strong π-π interaction during thermal annealing process. Enlarged magnification DF-STEM images of NPCNT-2 can be seen from Fig. 1c-e. A network structure with CNTs supported exfoliated graphene nano-ribbons can be seen from the overview DF-STEM image (Fig. 1c). Moreover, longitude opening "mouth" like structure (Fig. 1d-e) on CNTs indicated the longitude unzipping of MWCNTs. The unique opening structure enables the electrolyte easily entering the CNTs and facilitated the contact between oxygen and electrolyte. However, the mechanical incorporating of MWCNTs with graphene layers is difficult to form a uniform graphene layer-MWCNTs-graphene layer structure via a mechanical incorporating method. Therefore, this unique stereo structure obtained by partial exfoliation of MWCNTs would combine the high electro-conductivity of CNTs and ultra-high specific surface area of graphene nanoribbons together which is better than the simple mechanical incorporating MWCNTs with graphene layers. The DF-STEM image and the corresponding energy dispersive spectrometer (EDS) elemental distribution analysis in Fig. 2a-d shows homogenous distribution of C, N and P element. Due to the high annealing temperature of 900 ℃, NH4H2PO4 would experience a complete decomposition at the extreme temperature condition. Therefore, N and P elements were successfully doped into the carbon structure.

|
Download:
|
| Fig. 1.(a) TEM image of NPCNT-0, (b) DF-STEM image of NPCNT-4 and (c–e) the enlarged DF-STEM images of NPCNT-2. | |
|
Download:
|
| Fig. 2.(a) DF-TEM image of NSCNT-2 with the corresponding carbon (b), nitrogen (c) and phosphorus (d) of elemental mapping images, respectively. | |
The surface elemental composition of NPCNT-2 was determined by XPS analysis. Fig. 3a-c shows the high-resolution XPS spectrum of C 1s, N 1s and P 2p, respectively. The C 1s spectrum was deconvoluted into four single peaks which present C-C bond (284.6 eV), C-P bond (285.5 eV), C-N bond (287.2 eV) and COOH (289.0 eV) bond, further confirming that N and P atoms have been successfully doped into the stereo framework [19, 20]. The highresolution N1s spectrum revealed three single N species peaks at 398.7 eV, 399.8 eV and 401.0 eV which correspond to pyridinic N, pyrrolic N and graphitic N, respectively [21]. Recent researches have been recognized that graphitic N and pyridinic N play critic roles in determining the ORR performance of the carbon based materials [5]. Graphitic N mainly accounts for the conductivity and determines the limiting current density, while pyridinic N leads to the interaction of oxygen and controls the onset potential. Moreover, NPCNT-2 containing P atoms deconvoluted into two characteristic peaks shown at 132.8 eV and 134.1 eV, attributing to the C-P bond and P-O bond, respectively [22, 23]. Raman spectra of all three samples in Fig. 3d shows the characteristic D band, G band and 2D band located at 1350 ± 20 cm-1, 1575 ± 20 cm-1 and 2750 ± 20 cm-1, respectively, which have been reported to be signatures of graphitic carbon materials [24]. The G band and 2D band are attributed to the first order scattering of the E2g mode of sp2 carbon atoms while the D band is induced by defects or disorder [25]. The intensity ratio (ID/IG) of NSCNT-0, NSCNT-2 and NSCNT-4 are calculated to be 0.90, 0.97 and 1.11, respectively. The increase of ID/IG is due to the partial exfoliation of MWCNT which produces lots of defects. Moreover, the G band of NSCNT-2 and NSCNT-4 slightly shift towards higher frequency with respect to that of NSCNT-0, indicating the successfully doping of heteroatoms in the composite [26, 27].

|
Download:
|
| Fig. 3.High-resolution XPS spectrum of C 1s (a), N 1s (b) and P 2p (c) for NPCNT-2 and (d) Raman spectra of NPCNT-0, NPCNT-2 and NPCNT-4. | |
The electrocatalytic performance of the NPCNT samples towards ORR was first studied via cyclic voltammetry (CV) in N2- and O2-saturated 0.1 mol L-1 KOH solution. Oxygen reduction peaks can be obviously seen in O2- saturated solution when compared with the CV curves in N2-saturated solution (Fig. 4a). Moreover, NPCNT-2 showed more positive oxygen reduction peak potential (0.76 V) than NPCNT-0 and NPCNT-4 samples (0.65 V and 0.74 V, respectively), indicating excellent electrocatalytic activity of the NPCNT-2 towards ORR. To better understand the ORR catalytic activity of the samples, linear sweep voltammetry (LSV) measurement was conducted and compared with Pt/C catalyst. Among the investigated samples, the NPCNT-2 displayed the best ORR activity in terms of onset and half wave potentials (0.90 V and 0.77 V vs. RHE) which was slightly lower than commercial Pt/C (0.99 V and 0.78 V,Fig. 4b), while the NPCNT-0 and NPCNT-4 showed poor onset and half wave potential and limiting diffusion current density. Therefore, we can conclude that a moderate exfoliation of MWCNT resulting in the remaining carbon nanotube skeleton supported the exfoliated graphene ribbons, forming a three dimensional structure, which promoted the limiting diffusion current density of NPCNT-2 (equal to Pt/C). However, fully or inadequate exfoliation would result in poor catalytic activity. The poor performance of NPCNT-4 was probably caused by the agglomeration of graphene ribbons after high temperature calcination procedure. The Tafel plots for ORR on the three samples and Pt/C were calculated by plotting the logarithm of the kinetic current density derived from the LSV curves of Fig. 4b to further investigate the electrode kinetics. As shown in Fig. 4c, the Tafel slope on NPCNT-2 is ~46.7 mV dec-1, which is much lower than that of NPCNT-0 (~57.8 mV dec-1), NPCNT-4 (~50.0 mV dec-1) and Pt/C (~92.3 mV dec-1), indicating a more favourable ORR kinetic on NPCNT-2. The Tafel slope in low overpotential indicated the first electron transfer step by breaking the O-O bond is probably the rate determining step in a direct 4-electron O2 reduction [28, 29, 30]. Therefore, the lower Tafel slope of NPCNT-2 undergoes a faster ORR rate.

|
Download:
|
| Fig. 4.(a) CV curves of NPCNT samples at a scan rate of 50 mV s-1 in N2- and O2- saturated0.1mol L-1KOH solution, (b) ORRpolarizationcurves of NPCNT samples and Pt/C at a rotating speed of1600 rpmin O2-saturated 0.1mol L-1KOH solution, (c) Tafel plots of ORR polarization curves of (b), (d) ORR polarization curves of NPCNT-2 in O2-saturated 0.1mol L-1 KOH solution at different rotating speed, (e) Koutecky– Levich plots under potentials at 0.65 V, 0.70 V and 0.75 V and (f) i-t chronoamperometric methanol tolerance measurement of NPCNT-2 and Pt/C at potential of 0.7 Vby injecting 1 mol L-1 methanol at 400 s in 0.1 mol L-1 KOH solution. | |
The polarization curves for ORR with different rotation speeds are shown in Fig. 4d. The ORR polarization curves show a diffusioncontrolled region at voltages lower than 0.6 V, a diffusion-kinetic combined region (0.6-0.8 V), and a kinetic-controlled region at voltages higher than 0.8 V (vs. RHE). The corresponding Koutecky- Levich plots (j vs. ω-1/2) in Fig. 4e constructed from Eqs. (1) and (2) show that diffusion current linearly scale with v-1/2 and demonstrate a first-order ORR kinetics at potentials of 0.65 V, 0.70 V and 0.75 V [6]. The electron transfer number (n) of NPCNT-2 was quantitatively calculated to be 3.82, 3.97 and 3.94 (at the above three potentials, respectively) by using the slopes of these plots, indicating a 4-electron pathway dominated ORR process.
Fuel crossover effect is an important issue challenging the cathode catalysts in current fuel cell techniques [31, 32]. The methanol tolerance of NPCNT-2 and Pt/C catalysts towards ORR were evaluated through chronoamperometric measurements at constant potential of 0.7 V and rotation speed of 1600 rpm. When injected 1 mol methanol into the solution at 400 s (Fig. 4f), NPCNT- 2 showed slight fluctuation and remains stable current response while Pt/C suffered a sharp loss of 23.5% activity. These results indicate the better methanol tolerance of NPCNT-2 than Pt/C catalyst which is suitable as cathode catalyst for alkaline fuel cells.
4. ConclusionIn summary, N and P co-doped 3-D structured graphene nanoribbons/CNTs composite was successfully prepared by unzipping of MWCNTs and following the heat-treatment with ammonium dihydrogen phosphate. The unique structure reserved both the properties of carbon nanotube and graphene, exhibited excellent catalytic performance for the ORR with excellent onset and half-wave potential when compared with NPCNT-0, NPCNT-4 and Pt/C. Moreover, NPCNT-2 catalyst showed much better methanol tolerance in comparison to Pt/C.
Acknowledgments This work was supported by the National Natural Science Foundation of China (Nos. 21306060, 21573083), the Program for New Century Excellent Talents in University of Ministry of Education of China (No. NCET-13-0237), the Doctoral Fund of Ministry of Education of China (No. 20130142120039), the Fundamental Research Funds for the Central University (Nos. 2013TS136, 2014YQ009). We thank Analytical and Testing Center of Huazhong University of Science and Technology for allowing us to use its facilities. S/TEM work was carried out at the Center for Functional Nanomaterials, Brookhaven National Laboratory, which is supported by the U.S. Department of Energy, Office of Basic Energy Sciences (No. DE-SC0012704).
| [1] | Y.J. Si, C.G. Chen, W. Yin, H. Cai, Electrocatalytic activity of non-precious metal catalyst Co-N/C toward oxygen reduction reaction, Chin. Chem. Lett. 21(2010) 983-986. |
| [2] | B. Zheng, J. Wang, F.B. Wang, X.H. Xia, Synthesis of nitrogen doped graphene with high electrocatalytic activity toward oxygen reduction reaction, Electrochem. Commun. 28(2013) 24-26. |
| [3] | S.Y. Wang, D.S. Yu, L.M. Dai, D.W. Chang, J.B. Baek, Polyelectrolyte-functionalized graphene as metal-free electrocatalysts for oxygen reduction, ACS Nano 5(2011) 6202-6209. |
| [4] | P. Chen, T.Y. Xiao, Y.H. Qian, S.S. Li, S.H. Yu, A nitrogen-doped graphene/carbon nanotube nanocomposite with synergistically enhanced electrochemical activity, Adv. Mater. 25(2013) 3192-3196. |
| [5] | J. Liang, X. Du, C. Gibson, X.W. Du, S.Z. Qiao, N-doped graphene natively grown on hierarchical ordered porous carbon for enhanced oxygen reduction, Adv. Mater. 25(2013) 6226-6231. |
| [6] | Z.H. Sheng, H.L. Gao, W.J. Bao, F.B. Wang, X.H. Xia, Synthesis of boron doped graphene for oxygen reduction reaction in fuel cells, J. Mater. Chem. 22(2012) 390-395. |
| [7] | Y.H. Cheng, Y.Y. Tian, X.Z. Fan, J.G. Liu, C.W. Yan, Boron doped multi-walled carbon nanotubes as catalysts for oxygen reduction reaction and oxygen evolution reactionin in alkaline media, Electrochim. Acta 143(2014) 291-296. |
| [8] | T. Xing, Y. Zheng, L.H. Li, et al., Observation of active sites for oxygen reduction reaction on nitrogen-doped multilayer graphene, ACS Nano 8(2014) 6856-6862. |
| [9] | Y.G. Li, W. Zhou, H.L. Wang, et al., An oxygen reduction electrocatalyst based on carbon nanotube-graphene complexes, Nat. Nanotechnol. 7(2012) 394-400. |
| [10] | H.S. Zhai, L. Cao, X.H. Xia, Synthesis of graphitic carbon nitride through pyrolysis of melamine and its electrocatalysis for oxygen reduction reaction, Chin. Chem. Lett. 24(2013) 103-106. |
| [11] | Y.J. Si, Z.P. Xiong, C.G. Chen, P. Liu, H.J. Wu, A non-precious metal catalyst for oxygen reduction prepared by heat-treating a mechanical mixture of carbon black, melamine and cobalt chloride, Chin. Chem. Lett. 24(2013) 1109-1111. |
| [12] | C.Z. Zhang, N. Mahmood, H. Yin, F. Liu, Y.L. Hou, Synthesis of phosphorus-doped graphene and its multifunctional applications for oxygen reduction reaction and lithium ion batteries, Adv. Mater. 25(2013) 4932-4937. |
| [13] | J. Wu, C. Jin, Z.R. Yang, J.H. Tian, R.Z. Yang, Synthesis of phosphorus-doped carbon hollow spheres as efficient metal-free electrocatalysts for oxygen reduction, Carbon 82(2015) 562-571. |
| [14] | Z. Yang, Z. Yao, G.F. Li, et al., Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction, ACS Nano 6(2011) 205-211. |
| [15] | Y.J. Zhang, M. Chu, L. Yang, et al., Synthesis and oxygen reduction properties of three-dimensional sulfur-doped graphene networks, Chem. Commun. 50(2014) 6382-6385. |
| [16] | Z. Yao, H.G. Nie, Z. Yang, et al., Catalyst-free synthesis of iodine-doped graphene via a facile thermal annealing process and its use for electrocatalytic oxygen reduction in an alkaline medium, Chem. Commun. 48(2012) 1027-1029. |
| [17] | Y. Zhao, L.J. Yang, S. Chen, et al., Can boron and nitrogen co-doping improve oxygen reduction reaction activity of carbon nanotubes? J. Am. Chem. Soc. 135(2013) 1201-1204. |
| [18] | D.V. Kosynkin, A.L. Higginbotham, A. Sinitskii, et al., Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons, Nature 458(2009) 872-876. |
| [19] | S. Chen, J.Y.Bi,Y.Zhao, etal.,Nitrogen-dopedcarbon nanocages as efficientmetal-free electrocatalysts for oxygen reduction reaction, Adv. Mater. 24(2012) 5593-5597. |
| [20] | D.S. Yu, Y.H. Xue, L.M. Dai, Vertically aligned carbon nanotube arrays co-doped with phosphorus and nitrogen as efficient metal-free electrocatalysts for oxygen reduction, J. Phys. Chem. Lett. 3(2012) 2863-2870. |
| [21] | G. Wu, K.L. More, C.M. Johnston, P. Zelenay, High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt, Science 332(2011) 443-447. |
| [22] | J. Wu, Z.R. Yang, X.W. Li, et al., Phosphorus-doped porous carbons as efficient electrocatalysts for oxygen reduction, J. Mater. Chem. A 1(2013) 9889-9896. |
| [23] | R. Li, Z.D. Wei, X.L. Gou, W. Xu, Phosphorus-doped graphene nanosheets as efficient metal-free oxygen reduction electrocatalysts, RSC Adv. 3(2013) 9978-9984. |
| [24] | L.F. Chen, Z.H. Huang, H.W. Liang, H.L. Gao, S.H. Yu, Three-dimensional heteroatom-doped carbon nanofiber networks derived from bacterial cellulose for supercapacitors, Adv. Funct. Mater. 24(2014) 5104-5111. |
| [25] | J.L. Long, X.Q. Xie, J. Xu, et al., Nitrogen-doped graphene nanosheets as metal-free catalysts for aerobic selective oxidation of benzylic alcohols, ACS Catal. 2(2012) 622-631. |
| [26] | D.H. Deng, X.L. Pan, L. Yu, et al., Toward N-doped graphene via solvothermal synthesis, Chem. Mater. 23(2011) 1188-1193. |
| [27] | F.X. Ma, J. Wang, F.B. Wang, X.H. Xia, The room temperature electrochemical synthesis of N-doped graphene and its electrocatalytic activity for oxygen reduction, Chem. Commun. 51(2015) 1198-1201. |
| [28] | Y.Q. Zhao, J.J. Liu, Y.H. Zhao, F. Wang, Composition-controlled synthesis of carbonsupported Pt-Co alloy nanoparticles and the origin of their ORR activity enhancement, Phys. Chem. Chem. Phys. 16(2014) 19298-19306. |
| [29] | B.B. Blizanac, P.N. Ross, N.M. Marković, Oxygen reduction on silver low-index single-crystal surfaces in alkaline solution:rotating ring diskAg(h k l) studies, J. Phys. Chem. B 110(2006) 4735-4741. |
| [30] | V. Stamenković, T.J. Schmidt, P.N. Ross, N. Marković, Surface composition effects in electrocatalysis:kinetics of oxygen reduction on well-defined Pt3Ni and Pt3Co alloy surfaces, J. Phys. Chem. B 106(2002) 11970-11979. |
| [31] | J.T. Jin, F.P. Pan, L.H. Jiang, et al., Catalyst-free synthesis of crumpled boron and nitrogen co-doped graphite layers with tunable bond structure for oxygen reduction reaction, ACS Nano 8(2014) 3313-3321. |
| [32] | J. Wang, H.L.L. Xin, J. Zhu, et al., 3D hollow structured Co2FeO4/MWCNT as an efficient non-precious metal electrocatalyst for oxygen reduction reaction, J. Mater. Chem. A 3 (2015) 1601-1608. |
 2016, Vol.27
2016, Vol.27 


