The spirooxindole system is the core structure of many pharmacological agents and natural alkaloids [1, 2]. Thus, spirooxindole has been attracted much interest in synthetic and medical chemistry [3, 4]. Among the diverse heterocyclic spirooxindole ring system, spiro[indoline-3, 4′-chromene] is one of the particular heterocyclic members because they possess important biological activities [5, 6, 7]. The conventional reaction for the synthesis of spiro[indoline-3, 4′-chromene] derivatives was the three-component reaction of isatin, malononitrile and reactive methylene compounds under various reaction conditions. The conventional procedure for this reaction was employing common organic bases such as triethylamine, piperidine, and DBU as base catalyst in organic solvents [8]. In order to develop more efficient and greener methods, many other catalysts such as surfactant TEBA (triethylbenzylammonium chloride) [9], NH4Cl [10], sodium stearate [11], mesoporous silica nanoparticles [12, 13], HAuCl4 [14], nanocrystalline MgO [15] and sulfated choline [16] have been employed in the reaction. Additionally, the two- and three-component reactions were also carried out in ionic liquid, microwave, and electrocatalytic reaction manner [17, 18]. Practically, the three-component reaction could be also successfully applied to α-naphthol, β-naphthol and resorcinol with isatin and malononitrile for preparing benzo-fused spiro[indoline-3, 4′-chromene] derivatives [19, 20, 21, 22, 23, 24, 25]. However, a literature survey indicated there is only one example describing the reaction of 8-hydroquinoline with isatin and ethyl cyanoacetate for the synthesis of quinoline-fused spiro[indoline-3, 4′-chromene] [26]. In continuation of our aim to explore more efficient multicomponent reactions for biological active spiroxondole systems [27, 28, 29, 30], herein we report our new investigation for developing the three-component reaction of 8-hydroquinoline, isatin and malononitrile to synthesis of densely substituted spiro[indoline-3, 4′-pyrano[3, 2-h]quinolines].
2. Experimental 2.1. General procedure for the three-component reaction8-Hydroxyquinoline (1.0 mmol), isatin (1.1 mmol) and malonitrile or ethyl cyanoacetate (1.0 mmol) was dissolved in ethanol (20.0 mL). Then piperidine (1.0 mmol) was added and the mixture was stirred at room temperature for about 12 h. The resulting precipitates were collected by filtration and washed with cold alcohol to give the pure product. 1 H NMR and 13C NMR spectra for all new compounds are available in Supporting information.
2.2. 2′-Amino-2-oxospiro[indoline-3, 4′-pyrano[3, 2-h]quinoline]-30-carbonitrile (1a)White solid, 85%, mp 210-212 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.71 (s, 1H, NH), 9.00-8.99 (m, 1H, ArH), 8.36 (d, 1H, J = 8.0 Hz, ArH), 7.67-7.62 (m, 2H, ArH), 7.49 (s, 2H, NH2), 7.31 (t, 1H, J = 15.2 Hz, ArH), 7.04-7.00(m, 2H, ArH), 6.68-6.66 (d, 1H, J = 8.4 Hz, ArH); 13C NMR (100 MHz, DMSO-d6): δ 179.1, 161.6, 151.0, 144.4, 142.3, 137.9, 136.5, 135.2, 129.8, 128.6, 125.1, 124.6, 124.3, 123.3, 123.1, 119.2, 118.9, 110.5,
54.5, 51.6; IR (KBr, cm-1): υ 3476, 3445, 3295, 3190, 30=, 2194, 1726, 1693, 1656, 1621, 1600, 1568, 1499, 1470, 1403, 1365, 1322, 1268, 1233, 1198,
1170, 1157, 1141, 1118, 1064, 1037, 989, 924, 871, 845, 834, 796, 752;MS (m/z):HRMS (ESI) Calcd. for C20H12N4NaO2 ([M+ Na]+): 363.0852; Found: 363.0860.
White solid, 89%, mp176-180 ℃; 1H NMR (400 MHz, DMSO-d6): δ 9.02-9.01 (m, 1H, ArH), 8.39 (d, 1H, J = 8.4 Hz, ArH), 7.70-7.66 (m, 2H, ArH), 7.64 (s, 2H, NH2), 7.41-7.30(m, 7H, ArH), 7.10-7.08(m, 1H, ArH), 6.64-6.62(m, 1H, ArH), 5.10 (d, 1H, J = 7.8 Hz, CH2), 4.95 (d, 1H, J = 8.0 Hz, CH2) 13CNMR (100MHz, DMSO-d6): δ 177.5, 161.8, 151.1, 144.6, 141.7, 138.0, 136.5, 136.2, 136.0, 129.8, 129.1, 128.9, 128.2, 128.0, 127.6, 125.7, 124.8, 124.0,
123.3, 118.9, 118.1, 53.6, 51.4, 43.8; IR (KBr, cm-1): y 3182, 3063, 3030, 2193, 1720, 1654, 1625, 1603, 1565, 1482, 1409, 1370, 1334, 12=, 1192, 1162, 1112, 1080, 1046, 987, 946,
924, 880, 858, 832, 814, 767;MS (m/z): HRMS (ESI) Calcd.for C27H17ClN4NaO2 ([M+ Na]+): 487.0932; Found 487.0933.
White solid, 78%, mp266-268 ℃; 1H NMR (400 MHz, DMSO-d6): δ 10.45 (s, 1H, NH), 8.98-8.97 (m, 1H, ArH), 8.33-8.31 (m, 1H, ArH), 8.16 (s, 2H, NH2), 7.64-7.57 (m, 2H, ArH), 7.00-6.98 (m, 1H, ArH), 6.83-6.75 (m, 3H, ArH), 3.80-3.75 (m, 2H, CH2), 2.13 (s, 3H, CH3), 0.81 (t, 3H, J = 7.4 Hz, CH3). 13C NMR (100 MHz, DMSO-d6): δ 181.1, 168.2, 161.4, 150.8, 143.1, 139.9, 139.7, 137.9, 136.3, 131.2, 128.6, 128.3, 124.6, 124.3, 124.2, 122.9, 121.0, 109.5,
73.8, 59.2, 51.1, 21.0, 13.7. IR (KBr, cm-1): υ 3545, 3409, 3300, 3033, 2981, 2902, 2865, 1708, 1679, 1580, 1527, 1491, 1408, 1367, 1303, 1264, 1241, 1111, 1064, 1025,
984, 947, 852, 830, 810, 791, 766;MS (m/z): HRMS (ESI) Calcd. for C23H20N3O4 ([M+ H]+): 402.1448; Found 402.1452.
White solid, 90%, mp146-148 ℃; 1H NMR (400 MHz, DMSO-d6): δ 8.95-8.94 (m, 1H, ArH), 8.30-8.28 (m, 1H, ArH), 8.22 (s, 2H, NH2), 7.62-7.54 (m, 2H, ArH), 7.32-7.30 (m, 1H, ArH) 7.16-7.07 (m, 2H, ArH), 6.61-6.58 (m, 1H, ArH), 3.79-3.76 (m, 2H, CH2), 3.67-3.58 (m, 2H, CH2), 1.65-1.62 (m, 2H, CH2), 1.42-1.35 (m, 2H, CH2), 0.91 (t, 3H, J = 7.2 Hz, CH3), 0.67 (t, 3H, J = 6.8 Hz, CH3). 13C NMR (100 MHz, DMSO-d6): δ 178.9, 167.9, 161.6, 150.9, 143.4, 142.3, 140.6, 137.9, 136.3, 128.5, 128.4. 127.0, 124.4, 123.9, 123.1, 119.7, 110.3, 72.9, 59.0, 50.6, 29.7, 20.2, 14.1. IR (KBr, cm-1): y 3539, 3401, 3270, 2995, 2952, 2929, 2869, 1710, 1667, 1644, 1614, 1581, 1529, 1481, 1430, 1409, 1376, 1339, 1288, 1226,
1205, 1169, 1105, 1034, 981, 928, 880, 827, 789, 773, 759; MS(m/z): HRMS (ESI) Calcd. for C26H25ClN3O4 ([M+ H]+): 478.1528; Found 478.1533.
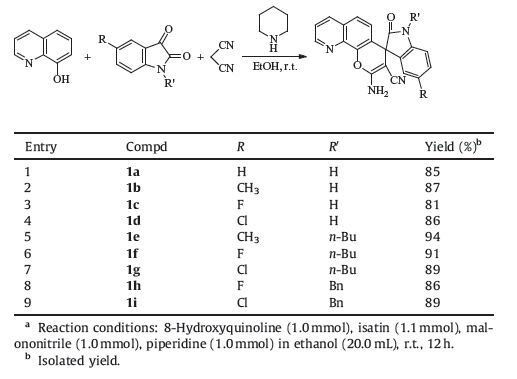
Initially, the reaction conditions of three-component reaction of 8-hydroquinoline, isatin and malononitrile were examined according to the previous reported procedure for the similar reaction of β-naphthol [20]. When the three-component reaction was carried out in ethanol at room temperature in the presence of equivalent common organic bases such as triethylamine, piperidine, DABCO and DBU, the expected spiro[indoline-3, 4′-pyrano[3, 2-h]quinoline] 1a was obtained in 70%, 85%, 75% and 63% yields, respectively. When less amount of piperidine was employed, the yield of product 1a was decreased, which showed that the piperidine was not simply described as a catalyst, but as a base promoter. Under this mild reaction conditions, various substituted isatins reacted smoothly to give the products 1a-1i in 81%-94% yields (Table 1). It should be pointed out that the products 1a-1i can be obtained in very pure state by simple filtration and washing with a little cold alcohol, and further separation with chromatography was not needed. The structures of obtained spiro compounds 1a-1i were fully characterized by IR, HRMS, 1 H NMR and 13C NMR spectra. The single crystal structures of the two spiro compounds 1b and 1e were also determined by Xray diffraction method (Fig. 1). Crystallographic data 1b (CCDC 1444403) and 1e (CCDC 1444404) have been deposited at the Cambridge Crystallographic Database Centre.
|
|
Table 1 Synthesis of spiro compounds 1a-1i via three-component reactiona. |

|
Download:
|
| Fig. 1.Single crystal structures of spiro compounds 1b and 1e. | |
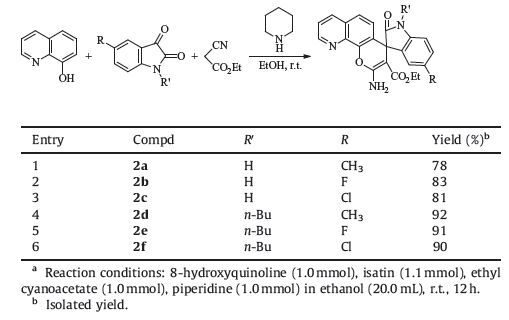
Then, ethyl cyanoacetate was also employed in the threecomponent reaction under samereactionconditions, the spiro[indoline- 3, 4′-pyrano[3, 2-h]quinoline]-3′-carboxylate 2a-2f were also produced in satisfactory yields (Table 2). Although the spiro compound 2a is a known compound [25], other products 2b-2f are newcompounds. The structures of spiro compounds 2a-2f were also established by spectroscopy. This result indicated that this three-component reaction has widely variety of substrates, from which diverse spiro[indoline-3, 4′-pyrano[3, 2-h]quinoline] derivatives can be conveniently synthesized.
|
|
Table 2 Synthesis of spiro compounds 2a-2f via three-component reactiona. |
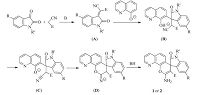
A brief reaction mechanism for three-component reaction was proposed in Scheme 1. At first, condensation of isatin with malononitrile resulted in isatylidene malononitrile (A) in the presence of piperidine. Second, Michael addition of the enolate of 8-hydroxyquinoline to isatylidene malononitrile (A) afforded a intermediate (B). Third, the phenolate ion (C) was regenerated by negative charge immigration. Then the intramolecular nucleophilic attack of phenolate to cyano group gave a dihydropyran ring intermediate (B), which in turn transferred to the final product 1 or 2 through amino-imine tautomerization process.
|
Download:
|
| Scheme 1.Proposed reaction mechanism for three-component reaction. | |
In summary, we have successfully developed a convenient procedure for the efficient synthesis of spiro[indoline-3, 4′- pyrano[3, 2-h]quinoline] derivatives by base promoted threecomponent reaction of 8-hydroxyquinoline, isatin and malononitrile or ethyl cyanoacetate. This reaction has the advantages of using readily available reagents, mild reaction conditions, simple separation procedure and high yields. This reaction might be found potential applications for the synthesis of the complex spiro heterocycles in synthetic and medicinal chemistry.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21172189, 21572196) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. We also thank the Analysis and Test Center of Yangzhou University providing instruments for analysis.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.02.016.
| [1] | B. Yu, D.Q. Yu, H.M. Liu, Spirooxindoles:promising scaffolds for anticancer agents, Eur. J. Med. Chem. 97(2015) 673-698. |
| [2] | S.A. Babu, R. Padmavathi, N.A. Aslam, V. Rajkumar, Recent developments on the synthesis and applications of natural products-inspired spirooxindole frameworks, Stud. Nat. Prod. Chem. 46(2015) 227-339. |
| [3] | G.S. Singh, Z.Y. Desta, Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks, Chem. Rev. 112(2012) 6104-6155. |
| [4] | M.M.M. Santos, Recent advances in the synthesis of biologically active spirooxindoles, Tetrahedron 70(2014) 9735-9757. |
| [5] | L. Bonsignore, G. Loy, D. Secci, A.Calignano, Detection of thehypothiocyanite (OSCN-) ion in human parotid saliva and the effect of pH on OSCN- generation in the salivary peroxidase antimicrobial system, Eur. J. Med. Chem. 28(1993) 517-520. |
| [6] | N. Yu, J.M. Aramini, M.W. Germann, Z. Huang, Reactions of salicylaldehydes with alkyl cyanoacetates on the surface of solid catalysts:syntheses of 4H-chromene derivatives, Tetrahedron Lett. 41(2000) 6993-6996. |
| [7] | Y.B. Wagh, Y.A. Tayade, S.A. Padvi, et al., A cesium fluoride promoted efficient and rapid multicomponent synthesis of functionalized 2-amino-3-cyano-4H-pyran and spirooxindole derivatives, Chin. Chem. Lett. 26(2015) 1273-1277. |
| [8] | Y.M. Litvinov, V.Y. Mortikov, A.M. Shestopalov, Versatile three-component procedure for combinatorial synthesis of 2-aminospiro[(3'H)-indol-3', 4-(4H)-pyrans], J. Comb. Chem. 10(2008) 741-745. |
| [9] | S.L. Zhu, S.J. Ji, Y. Zhang, A simple and clean procedure for three-component synthesis of spirooxindoles in aqueousmedium, Tetrahedron63(2007) 9365-9372. |
| [10] | M. Dabiri, M. Bahramnejad, M. Baghbanzadeh, Ammonium salt catalyzed multicomponent transformation:simple route to functionalized spirochromenes and spiroacridines, Tetrahedron 65(2009) 9443-9447. |
| [11] | L.M. Wang, N. Jiao, J. Qiu, et al., Sodium stearate-catalyzed multicomponent reactions for efficient synthesis of spirooxindole in aqueous micellar media, Tetrahedron 66(2010) 339-343. |
| [12] | G.M. ZIarani, A. Badiei, S. Mousavi, N. Lashgari, A. Shahbazi, Application of aminofunctionalized sba-15 type mesoporous silica in one-pot synthesis of spirooxindole, Chin. J. Catal. 33(2012) 1832-1839. |
| [13] | K. Sarrafi, Ebrahim Mehrasbi, A. Vahid, M. Tajbakhsh, Well-ordered mesoporous silica nanoparticles as a recoverable catalyst for one-pot multicomponent synthesis of 4H-chromene derivatives, Chin. J. Catal. 33(2012) 1486-1494. |
| [14] | M. Kidwai, A. Jahan, N.K. Mishra, Gold(Ⅲ) chloride (HAuCl4·3H2O) in PEG:a new and efficient catalytic system for the synthesis of functionalized spirochromenes, Appl. Catal., A:Gen. 425-426(2012) 35-43. |
| [15] | B. Karmakar, A. Nayak, J. Banerji, A clean and expedient synthesis of spirooxindole in aqueous media catalyzed over nanocrystalline MgO, Tetrahedron Lett. 53(2012) 5004-5007. |
| [16] | S.P. Satasia, P.N. Kalaria, J.R. Avalani, D.K. Raval, An efficient approach for the synthesis of spirooxindole derivatives catalyzed by novel sulfated choline based heteropolyanion at room temperature, Tetrahedron 70(2014) 5763-5767. |
| [17] | N. Azizi, S. Dezfooli, M.M. Hashemi, Greener synthesis of spirooxindole in deep eutectic solvent, J. Mol. Liq. 194(2014) 62-67. |
| [18] | M.N. Elinson, A.I. Ilovaisky, A.S. Dorofeev, et al., Electrocatalytic multicomponent transformation of cyclic 1, 3-diketones, isatins, and malononitrile:facile and convenient way to functionalized spirocyclic (5, 6, 7, 8, tetrahydro-4H-chromene)-4, 3'-oxindole system, Tetrahedron 63(2007) 10543-10548. |
| [19] | G. Shanthi, G. Subbulakshmi, P.T. Perumal, A new InCl3-catalyzed, facile and efficient method for the synthesis of spirooxindoles under conventional and solvent-free microwave conditions, Tetrahedron 63(2007) 2057-2063. |
| [20] | H.R. Safaei, M. Shekouhy, S. Rahmanpura, A. Shirinfeshana, Glycerol as a biodegradable and reusable promoting medium for the catalyst-free one-pot three component synthesis of 4H-pyrans, Green Chem. 14(2012) 1696-1704. |
| [21] | R.Y. Guo, Z.M. An, L.P. Mo, et al., Meglumine:a novel and efficient catalyst for onepot, three-component combinatorial synthesis of functionalized 2-amino-4Hpyrans, ACS Comb. Sci. 15(2013) 557-563. |
| [22] | J.H. Park, Y.R. Lee, S.H. Kim, A novel synthesis of diverse 2-amino-5-hydroxy-4Hchromene derivatives with a spirooxindole nucleus by Ca(OH)2-mediated threecomponent reactions of substituted resorcinols with isatins and malononitrile, Tetrahedron 69(2013) 9682-9689. |
| [23] | H.R. Shaterian, F. Rigi, New applications of cellulose-SO3H as a bio-supported and biodegradable catalyst for the one-pot synthesis of some three-component reactions, Res. Chem. Intermed. 40(2014) 2983-2999. |
| [24] | M. Daraie, Y.S. Beheshtiha, M.M. Heravi, Synthesis of spirochromene derivatives catalyzed by Mn(bpyo)2/MCM-41 in water, Monatsh. Chem. 146(2015) 191-198. |
| [25] | B. Sadeghi, M.G. Pirbaluti, P.F. Nezhad, R.A. Nezhad, A clean and expedient synthesis of spirooxindoles catalyzed by silica-sulfuric acid nanoparticles as an efficient and reusable reagent, Res. Chem. Intermed. 41(2015) 4047-4055. |
| [26] | F.F. Abdel-Latif, R.A. Mekheimer, M.M. Mashaly, E. Kh. Ahmed, The synthesis of heterocycles from indolin-2-one derivatives and active methylene reagents, Collect. Czech Chem. Commun. 59(1994) 1235-1240. |
| [27] | H. Gao, J. Sun, C.G. Yan, Selective synthesis of functionalized spiro[indoline-3, 2'-pyridines] and spiro[indoline-3, 4'-pyridines] by Lewis acid catalyzed reactions of acetylenedicarboxylate, arylamines, and isatins, J. Org. Chem. 79(2014) 4131-4136. |
| [28] | C. Wang, Y.H. Jiang, C.G. Yan, Convenient synthesis of spiro[indoline-3, 40-pyrano[2, 3-c]pyrazole] and spiro[acenaphthyl-3, 40-pyrano[2, 3-c]pyrazoles] via four-component reaction, Chin. Chem. Lett. 26(2015) 889-893. |
| [29] | H. Gao, J. Sun, C.G. Yan, Synthesis of new type of Betti bases via three-component reaction of β-naphthol, cyclic amines and isatins, Chin, Chem. Lett. 26(2015) 353-356. |
| [30] | G.L. Shen, J. Sun, C.G. Yan, Diastereoselective synthesis of spiro[benzo[d]pyrrolo-[2, 1-b]thiazole-3, 3'-indolines] via cycloaddition reaction of N-phenacylbenzothiazolium bromides and 3-methyleneoxindoles, Org. Biomol. Chem. 13(2015) 10929-10938. |
 2016, Vol.27
2016, Vol.27