b State Key Laboratory of Elemento-Organic Chemistry, Research Institute of Elemento-Organic Chemistry, Nankai University, Tianjin 300071, China
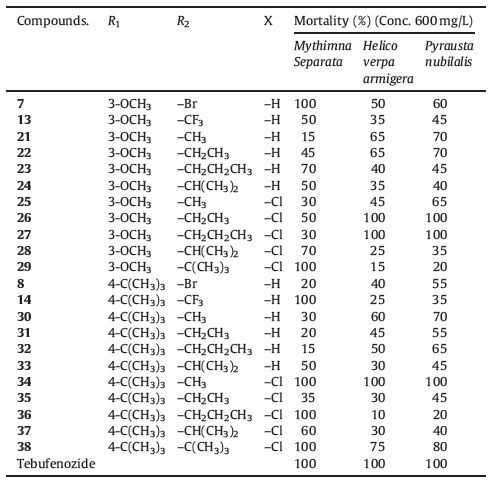
Molting hormones, such as 20-hydroxyecdysone (20E), are compounds that regulate the molting of insects [1, 2]. Molting or ecdysis is a critical aspect of insect growth. The binding of 20E to the ecdysone (EcR)-ultraspiracle protein (USP) heterodimer during insect development results in ecdysis. One prominent approach toward the development of environmentally benign insect growth regulators has been to design ligands that target the EcR-USP heterodimer. For example, the non-steroidal ecdysone agonists, dibenzoylhydrazines (DBHs), bind to the EcR subunit of the heterodimer and induce insect moulting. These agents, which bear the common dibenzoylhydrazine core structure, display outstanding activity against lepidopteran pests while having no negative effects on mammals and the environment [3, 4, 5, 6].
Recently, a series of novel ecdysone agonists with non-steroidal or non-dibenzoylhydrazine structures and displaying highly insecticidal activity have been identified [7, 8]. These ecdysone agonists have been rationally designed using target structureguided approaches. Additional efforts that include structural modifications of previously identified agonists may led to the identification of other highly active and novel ecdysone agonists in recent years [9] (I, Fig. 1). Hence, that modification of the structure designed by target ecdysone receptor is a success way to design new ecdysone agonists for pest controls.
|
Download:
|
| Fig. 1.Chemical structures of known potential ecdysone agonists. | |
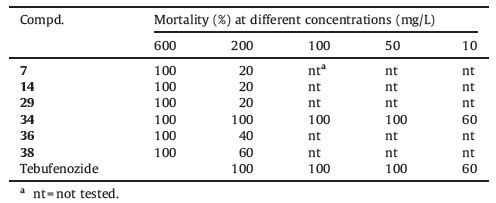
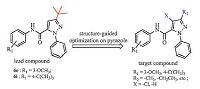
In the course of our research efforts, we had previously identified two compounds, 6e and 6i (Ⅱ, Fig. 1), which displayed high ecdysone agonistic activities, and an active conformer of these compounds was similar to that of tebufenozide [10]. Attribute to the hydrophobic effect of the t-butyl and methoxy substituent in phenyl ring and their excellent activity of 6e and 6i, so we retained themethoxyandt-butyl substituentsonaromatic amide atpositions 3 and 4, and then modified the substituent groups in the pyrazole ring. Inspired by these facts, we focused on designing and synthesizing novel substituted pyrazole amide derivatives based on the lead compounds 6e and 6i in this study to discover highly active ecdysone analogs (Fig. 2) and a range of lepidopteran biological activities was ascribe to this series. Additionally, selected examples of molecular docking and molecular dynamics studies were evaluated to study the mechanisms of these derivatives with active site of the EcR subunit of the heterodimeric receptor.
|
Download:
|
| Fig. 2.Design strategy of target compounds 7-8, 13-14 and 21-38 through modifying the substituent on pyrazole of lead 6e and 6i. | |
Melting points of all compounds were determined on an X-5 binocular (Fukai Instrument Co., Beijing, China), and were not corrected. 1H NMR spectra and 13C NMR were recorded on a Bruker AM-300 (300 MHz) spectrometer with CDCl3 or DMSO-d6 as the solvent and TMS as the internal standard. Chemical shifts were reported in δ (parts permillion) values. IR spectra were recorded on a Perkin Elmer Spectrum 100 FT-IR spectrometor (KBr presser method). High resolution mass spectrometry (HRMS) data were obtained on an FTICR-MS Varian 7.0 T FTICR-MS instrument. All the reagents were obtained commercially and used after further purification.
2.1. General procedures for synthesis and Insecticidal test of compounds 7-8, 13-14 and 21-38The general procedures for synthesis of compounds 7-8, 13-14 and 21-38 were listed in the Supporting information. Their biological activities against Mythimna separate, Helicoverpa armigera and Pyrausta nubilalis were evaluated using the reference methods [11, 12].
2.2. Molecular docking studyCompounds 6e, 6i, 34 and tebufenozide were built and optimized using the MMFF94 force field and charges in Molecular Operating Environment (MOE) software [13]. The first low energy conformation of every compound was selected to dock into the active pocket using the Induced Fit protocol. A crystal structure of ecdysone receptor (EcR) complexed with BYIO8346 (PDB ID: 1R20) was modified and protonated. Waters and phosphatidylethanolamines were deleted. The active site was set by the residues with a radius of 6 Å around BYIO8346. The docked small molecules were placed in the site with the Triangle Matcher method and ranked with the London dG scoring function. Thirty conformations with the docking poses were output and further rescored with the GBVI/ WSA dG scoring function. The docking complex with the rational binding mode and higher scoring would be studied further for molecular dynamics simulation.
2.3. Molecular dynamics simulationThe molecular dynamics (MD) simulation were finished using the AMBER 12 software [14]. EcR receptor was solvated using explicit TIP3P water models and the water molecules were filled in the range of 10 Å bound to the protein atomin a cubic periodic box.
AMBER ff99SB force field was used for EcR and gaff force field was assigned to the small molecules. Energy minimization was first performed using the steepest descent algorithm for two thousand steps and then the conjugated gradient algorithm for another three thousand steps before the MD simulation. Then three dynamics simulation steps were applied to the whole system. First, only waters and counterions were heated for 10 ps to ensure the solute was entirely solvated; second, the system was slowly heated from 10 K to 298 K by a weak-coupling method and equilibrated for 100 ps except the protein backbone; finally, the full system was executed to a constant pressure equilibration for 20 ns. The binding free energies between ligands and EcR were computed, as a sum of the gas-phase molecular mechanics energies, and solvation energies, and conformational entropy, based on the MM-PBSA method.
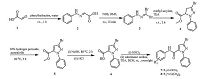
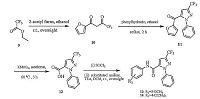
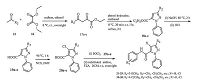
3. Results and discussionThe synthetic procedures for substituted pyrazole amide derivatives are depicted in Schemes 1-3. Condensation of the phenylhydrazine with malonaldehydic acid 1 yields the compound 2. Bromination of 2 with NBS gives the compound 3, which undergoes cyclization with a methyl acrylate to afford the bromopyrazoline 4 [15]. The oxidation of 4 affords the pyrazole ester 5 [16], which is subjected to saponification and amidation to afford the target amides 7 and 8 in moderate overall yields (Scheme 1). A different method is followed to install a trifluoro methyl group on the pyrazole ring (Scheme 2). The 1, 3-diketone compound 10 is synthesized from 9 following a previously described protocol [17]. The trifluromethyl-substituted pyrazole core of the key intermediate 11 is prepared by the cyclization of the 1, 3-diketone moiety in 10 with phenylhydrazine [17]. Subsequently, oxidation of 11 and the amidations of the resulting carboxylic acid 12 yield the desired trifluromethyl-substituted pyrazole amides 13 and 14 in moderate yields [18]. The synthesis of the alkyl-substituted pyrazoles 21-38 is initiated with the compounds 15 following a previously described protocol [19] (Scheme 3). The pyrazole core of the key intermediate 18a-e is prepared by the cyclization of the 1, 3-diketone moiety in 17a-e with phenylhydrazine (Scheme 3).The saponification of the ester 18a-e affords the carboxylic acid 19a-e, and aromatic halogenation of 19a-e gives the chlorine substituted compound 20a-e [20]. Amidations of the carboxylic acids (containing pyrazole and chloro-pyrazole motifs) using appropriately substituted anilines obtain the target compounds 21-38 in moderate yields.
|
Download:
|
| Scheme 1.Synthetic procedure for title compouds 7-8. | |
|
Download:
|
| Scheme 2.Synthetic procedure for title compounds 13-14. | |
|
Download:
|
| Scheme 3.Synthetic procedure for title compouds 21-38. | |
The structures of the targeted compounds are confirmed through the analyses of their melting points, 1 H NMR spectra, 13C NMR spectra, IR spectra, and HRMS. These data can be found in the Supporting information. Signals corresponding to the C-H and the N-H protons of the pyrazole ring in target compounds are observed at about δ ~ 6.84-6.68 and δ ~ 7.97-8.71 in the 1H NMR spectra of the compounds, respectively signals as the C-H protons of t-butyl substituent and methoxy substituent in phenyl ring are observed at about δ ~ 1.33 and δ ~ 3.79 and signal for the protons on the benzene ring are observed at δ 6.63-7.73. In the IR spectra of target compounds, strong absorptions at 3200-3400 cm-1, functionality, are observed. In addition, other strong absorptions bonds representing the carbonyl and unsaturated pyrazole groups are observed at 1600-1700 cm-1 and 1530 cm-1, respectively. The HRMS data of the target compounds are in good agreement with the theoretical data calculated based on the chemical formulae.
The insecticidal activities of all title compounds were evaluated against lepidopteran pests (M. separata, H. armigera, and P. nubilalis) in vivo and the corresponding data are showninTable 1. In this study, we focused on the relative small sizes and suitable hydrophobic effects of the substituents at the modification positions on the pyrazole ring. Therefore, a series of alkyl substitutes, trifluoro methyl group, and bromine atom at position 3 were taken into consideration, as well as hydrogen atom and chlorine atom at position 4.While keeping the methoxy on phenyl ring at positions 3, compounds 7 and 29, exhibited superior activity against M. separate. The compounds 26, 27 harboring ethyl or n-propyl substituents at position 3 and chlorine atom at position 4 on the pyrazole ring displayed promising activity against H. armigera, and P. nubilalis. Respectively, retaining the substituents as t-butyl at position 4 of the phenyl substituent, higher activity was observed with compound 14, 34, 36, and 38 against M. separate. Moreover, compound 34 even exhibited excellent activities against H. armigera, and P. nubilalis, containing 3-methyl and 4-chloro substituent on pyrazole ring. In particular, the superior activity of compound 34 against M. separata at lower doses was comparable to that displayed by the positive control tebufenozide (see Table 2). This indicates that different sizes of substituents on the pyrazole ring contribute significantly to the insecticidal activities in vivo.
|
|
Table 1 Observed in vivo insecticidal activity (mortality) of pyrazole analogs. |
|
|
Table 2 Dosage-dependent in vivo insecticidal activity (mortality) of 7, 14, 29, 34, 36, 38 and tebufenozide against Mythimna Separata. |
Molecular docking is an effective method used in structureguided design, due to its ability to elucidate the possible binding conformations for ligands to bind into EcR/USP receptor. Moreover, the characterization of binding process, such as hydrogen bonding interactions and hydrophobic effect, plays a critical role in rational design of ecdysone analogs [7, 8]. In order to gain insights into the mode of action of the substituted pyrazole ring, molecular docking was considered to study the interactions of these compounds with EcR. The binding conformation of compound 34 and tebufenozide with the important residues of EcR were shown in Fig. 3a and b. The carbonyl oxygen of the amide group for compound 34 established a hydrogen bonding interaction with Tyr408 (Fig. 3a). The residues Asn504, and Thr343 were formed in the hydrogen bonding interactions with tebufenozide (Fig. 3b). Tyr408 is the important residue to ensure small molecules to keep the inhibiting activity to EcR. The docking results showed that compound 34 may be the potential inhibitor to EcR and its docking conformation was similar as that of tebufenozide. To analyze the binding affinity of small molecules to EcR, molecular dynamics simulations were performed for EcR complexed with compound 34 and tebufenozide, respectively. The calculated binding free energy is -64.8 kJ/mol for the complex of compound 34 and EcR, which is nearly close to that (-69.66 kJ/mol) for the complex of tebufenozide and EcR (Fig. S1 in Supporting information). These data further showed compound 34 might have binding affinity with EcR. We will carry out the [3]PonA-labeled binding experiments as enzymatic assay testing EC50 on EcR/USP to confirm the above supposition in future work.

|
Download:
|
| Fig. 3.Hydrogen bonding interactions between the residues at the binding pocket of the EcR subunit and compounds (a) 34 and (b) tebufenozide. Carbons in tebufenozide and 34 are represented by blue spheres. Oxygens and nitrogen atoms in both the structures are displayed as red and dark blue solid spheres, respectively. H-bonds are indicated by green dotted lines. (c) The comparison of the binding conformation between tebufenozide (in brown), 6e (in pink) and 6i (in blue). (d) The comparison of the binding conformation between tebufenozide (in brown) and 34 (in red). | |
In addition, our previous study showed that the binding modes of 6e or 6i with the EcR subunit were similar to that of tebufenozide [10]. Here, we aligned and compared the equilibrating conformations for 6e, 6i and tebufenozide in the pocket of EcR (Fig. 3c). The pyrazole rings of 6e, 6i located in the hydrophobic region in the binding pocket, which had the similar position as the t-butyl substituent of tebufenozide at the binding pocket (red cycle). Moreover, the hydrophobic t-butyl group interacting with the hydrophobic residues of EcR is a key factor affecting the hydrophobic parameters of the ligands, which is also important to the binding affinity and activity in vivo of DBHs [21, 22]. The better in vivo activity observed for the 6e and 6i may also be attributed to the hydrophobic effect of the substituted pyrazole. Then, we analyzed the superimposed conformations between tebufenozide and 34. Fig. 3d showed that the pyrazole ring of 34 almost fully occupied in the position where the hydrophobic t-butyl group of tebufenozide exited (red cycle). So increasing the hydrophobic effect and considering the suitable bulk effect on pyrazole ring, such as 3-methyl and 4-chloro substituents on pyrazole ring, will be beneficial to the inhibiting activity to EcR.
4. ConclusionThrough this study, a series of novel substituted pyrazole amides derivatives were obtained by structure-guided optimization method in basis of the core structures of the two active compounds N-(3-methoxy phenyl)-3-(tert-butyl)-1-phenyl-1Hpyrazole- 5-carboxamide (6e) and N-(4-(tert-butyl)phenyl)-3- (tert-butyl)-1-phenyl-1H-pyrazole-5-carboxamide (6i). In particular, the preliminary assays for insecticidal activity indicated that one of the compounds of the series, 34, showed excellent activities against lepidopteran pests, such as M. separate, that was particularly comparable to that displayed by tebufenozide.Selected examples from molecule docking and molecular dynamics studies demonstrated that compound 34 could be a potential inhibitor against EcR/USP receptor which binding conformation was similar to that of tebufenozide. Particularly, the comparison of the binding conformations indicated the increasing of hydrophobic effect and the suitable substituent effects of pyrazole ring were essential for the inhibiting activity to EcR and activity in vivo. These esults offer valuable insights toward discovering new active ecdysone analogs by modifying the nature of the substituent groups on the pyrazole ring.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21272265) and the National High Technology Research and Development Program of China (No.2011AA10A204).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.02.009.
| [1] | L.I. Gilbert, Halloween genes encode P450 enzymes that mediate steroid hormone biosynthesis in Drosophila melanogaster, Mol. Cell. Endocrinol. 215(2004) 1-10. |
| [2] | Y. Nakagawa, V.C. Henrich, Arthropod nuclear receptors and their role in molting, FEBS J. 276(2009) 6128-6157. |
| [3] | K.D. Wing, Rh-5849, a nonsteroidal ecdysone agonist-effects on a drosophila cell-line, Science 241(1988) 467-469. |
| [4] | T.S. Dhadialla, R.K. Jansson, Non-steroidal ecdysone agonists:new tools for IPM and insect resistance management, Pestic. Sci. 55(1999) 357-359. |
| [5] | G.R. Carlson, T.S. Dhadialla, R. Hunter, et al., The chemical and biological properties of methoxyfenozide, a new insecticidal ecdysteroid agonist, Pest Manage. Sci. 57(2001) 115-119. |
| [6] | Y. Sawada, T. Yanai, H. Nakagawa, et al., Synthesis and insecticidal activity of benzoheterocyclic analogues of N'-benzoyl-N-(tert-butyl)benzohydrazide:Part 2. Introduction of substituents on the benzene rings of the benzoheterocycle moiety, Pest Manage. Sci. 59(2003) 36-48. |
| [7] | G. Holmwood, M. Schindler, Protein structure based rational design of ecdysone agonists, Bioorg. Med. Chem. 17(2009) 4064-4070. |
| [8] | T. Harada, Y. Nakagawa, T. Ogura, et al., Virtual screening for ligands of the insect molting hormone receptor, J. Chem. Inf. Model. 51(2011) 296-305. |
| [9] | T. Yokoi, S. Minami, Y. Nakagawa, H. Miyagawa, Structure-activity relationship of imidazothiadiazole analogs for the binding to the ecdysone receptor of insect cells, Pestic. Biochem. Physiol. 120(2015) 40-50. |
| [10] | X.L. Deng, L. Zhang, X.P. Hu, et al., Target-based design, synthesis and biological activity of new pyrazole amide derivatives, Chin. Chem. Lett. 27(2015) 251-255. |
| [11] | W.L. Dong, J.Y. Xu, L.X. Xiong, X.H. Liu, Z.M. Li, Synthesis, structure and biological activities of Some novel anthranilic acid asters containing N-pyridylpyrazole, Chin. J. Org. Chem. 27(2009) 579-586. |
| [12] | J.W. Zhang, Y.Q. Li, X.L. Yang, et al., Synthesis and bioactivities of nucleoside compounds containing substituted benzoyl carbamate thiourea, Chin. J. Org. Chem. 33(2013) 305-311. |
| [13] | Molecular Operating Environment (MOE), 2013.08, Chemical Computing Group Inc., 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada H3A 2R7, 2013. |
| [14] | D.A. Case, T.A. Darden, T.E. Cheatham Ⅲ, et al., AMBER 12, University of California, San Francisco, CA, 2012. |
| [15] | F. Foti, G. Grassi, F. Risitano, First synthesis of a bromonitrilimine. Direct formation of 3-bromopyrazole derivatives, Tetrahedron Lett. 40(1999) 2605-2606. |
| [16] | R.A. Berger, J.L. Flexner, Pesticidal compositions for coating plant propagation material containing anthranilamides, WO Patent 2003024222, 2003. |
| [17] | J.C. Sloop, C.L. Bumgardner, W.D. Loehle, Synthesis of fluorinated heterocycles, J. Fluorine Chem. 118(2002) 135-147. |
| [18] | M. Muthuppalaniappan, S. Viswanadha, S.K.V.S. Vakkalanka, Preparation of pyrazole derivatives as modulators of calcium release-activated calcium channel for treatment of non-small cell lung cancer, WO Patent 2011042797, 2012. |
| [19] | Y.F. Sun, H.L. Qiao, Y. Ling, et al., New analogues of (E)-b-farnesene with insecticidal activity and binding affinity to aphid odorant-binding proteins, J. Agric. Food Chem. 59(2011) 2456-2461. |
| [20] | H.A. Stefani, C.M.P. Pereira, R.B. Almeida, et al., A mild and efficient method for halogenation of 3,5-dimethyl pyrazoles by ultrasound irradiation using N-halosuccinimides, Tetrahedron Lett. 46(2005) 6833-6837. |
| [21] | A.C.S. Santos, C.M.R. Sant'Anna, 20-hydroxyecdysone receptor ligand binding domain:1. A semiempirical study of dibenzoylhydrazines selectivity, J. Mol. Struct.:THEOCHEM 585(2002) 61-68. |
| [22] | A. Kasuya, Y. Sawada, Y. Tsukamoto, et al., Binding mode of ecdysone agonists to the receptor:comparative modeling and docking studies, J. Mol. Model. 9(2003) 58-65. |
 2016, Vol.27
2016, Vol.27