b Department of Plant Pathology, College of Agronomy and Biotechnology, China Agricultural University, Beijing 100193, China
Natural products (NPs) continue to play a highly significant role in the drug discovery and development process [1]. Natural products and their analogs, derivatives account for almost half of the 877 approved drugs all over the world from 1981 to 2010 [2]. In fact, exploring a range of natural product sources is also an important means to identify and develop novel lead compounds and agrochemicals against a range of insect pest and plant diseases in the recent decades. 1, 5-Diphenyl-2-penten-1-one (Ⅰ) and 1, 5- diphenylpentan-1-one (Ⅱ) (Fig. 1) were first isolated from Stellera chamaejasme L. (Thymelaeaceae, used in Chinese traditional medicine) in 2001 [3]. Laboratory bioassay showed that these two compounds had strong contact activity and very good antifeedant activity against Aphis gossypii and Schizaphis graminum. Moreover, compound I exhibited the similar effects on ATP-ase found in the three membranes amongst which the plasma membrane Ca2+-Mg2+-ATPase is the primary target [4, 5]. After that, various analogs with different bioactivities were synthesized by Hou’s group [6, 7, 8]. Our team is also devoted to the structural modification of Ⅰ and Ⅱ in the previous study. We found, beside insecticidal activity, compounds Ⅰ, Ⅱ and their analogs also have antifungal activities [9, 10, 11, 12], which indicated that they could be an interesting lead structure of fungicide.

|
Download:
|
| Fig. 1.The chemical structure of compounds Ⅰ and Ⅱ, originally isolated from Stellera chamaejasme L. | |
In continuation of our earlier interest in this field, here it was planned to synthesize new analogs by introducing 2, 6-dimethylmorpholine moiety, a functional group of commercial fungicide fenpropimorph, tridemorph, dodemorph, etc., to replace one side phenyl group of compound I, with expectation to obtain the new products with simple structure and better antifungal activities. The two isomers of target compounds were separated and identified by NOESY technique and chemical method. Their antifungal activities against six plant pathogenic fungi were evaluated for the first time. It is expected that the results of this study might be valuable for the discovery of new molecules as agrochemicals.
2. Experimental1 H NMR spectra were collected on Bruker AM-300 (300 MHz) spectrometer with CDCl3 as the solvent and TMS as the internal standard. IR spectra were recorded on a Perkin Elmer Spectrum 100 FT-IR spectrometer with a KBr disk. High resolution mass spectrometry (HRMS) data were recorded on an FTICR-MS Varian 7.0 T FTICR-MS instrument.Elemental analysiswasdeterminedonan ST-Carloerba. Co elemental analyser. All the reagents were obtained commercially and used without further purification. Column chromatography purification was carried out by using silica gel.
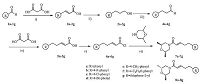
The general synthetic scheme for representative compounds 7a-7g, 8a-8g is shown in Scheme 1. Substituted cinnamic acids 2 were prepared from substituted benzaldehyde 1 through knoevenagel reaction according to the method in the literature [13]. Compounds 2a-2g were reduced by lithium aluminum hydride (LiAlH4) to afford substituted phenylpropanol 3a-3g. Next, compounds 3a-3g were oxidized by PCC to afford substituted benzenepropanal 4. (E)-5-(Substituted phenyl)pent-2-enoic acids 5 were obtained through the same process as compound 2. The general procedure of compounds 2a-2g to 5a-5g is described in the Supporting information.
|
Download:
|
| Scheme 1.Synthetic route of title compounds. (i) piperidine, pyridine, 85 ℃, 6 h;(ii) LiAlH4, THF, reflux, 4 h;(iii) PCC, CH2Cl2, r.t. 1 h;(iv) piperidine, pyridine, 85 ℃, 6 h; (v) SOCl2, DMF, CHCl3, reflux 1 h; (vi) TEA, CH2Cl2, r.t. 2 h. | |
Target compounds 7a-7g and 8a-8g were prepared by the acylchloriration of compounds 5a-5g followed by a condensation reaction with 2, 6-dimethylmorpholine at the presence of triethylamine (TEA). The general procedure describe as below: To a stirred solution of compounds 5 (6 mmol) in chloroform, thionyl chloride (18 mmol) and one to two drop of N, N-dimethyl formamide (DMF) was added, the resulted mixture was refluxed for 1 h. Then the solvent and remaining thionyl chloridewas removed under reduced pressure, the residuewasdissolvedin dichloromethane (DCM) to get the stock solution of 6 without further purification. The solution of 6 was dropped slowly into the solution of 2, 6-dimethylmorpholine (6 mmol) and TEA (6.6 mmol) in DCM at 0 ℃, the resulted mixture was stirred for 1 h. After the reaction completed, the mixture was washed with water. Organic layer was dried over anhydrous MgSO4 and concentrated in vacuo. Then the residue was separated by chromatography on silica-gel column (n-hexane:ethyl acetate = 4:1, v/v) to obtain the corresponding 7a-7g, 8a-8g.
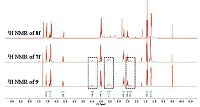
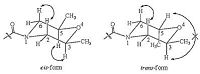
3. Results and discussionAs shown in Scheme 1, the target compounds 7a-7g, 8a-8g were synthesized via six steps, including Knoevenagel reaction, reduction, oxidation, and amidation, with substituted benzaldehyde as starting material. Characterization data of all the target compounds are included in the Supporting information. The material 2, 6-dimethylmorpholine we used in the last step was consisted of a mixture of cis-form and trans-form, due to the two methyl group could be on the same side or the different side of morpholine ring, namely cis-2, 6-dimethylmorpholine and trans- 2, 6-dimethylmorpholine. Therefore we obtained our target compounds with two isomers (7 and 8) as well, which can be separated easily by silica-gel column but very complicated to be identified. Clear difference could be seen on the 1H NMR spectra of two representative compounds 7f and 8f (Fig. 2). First, NOESY technique was used to identify the two isomers. As shown in Fig. 3, in the case of cis-form, there are two equatorial CH3-groups at 2, 6- position, NOEs between the protons of H2ax and H6ax, H3ax and H5ax should occur. While in the case of trans-form, there is one equatorial and one axial CH3-group, NOE should only occur between the protons of H2ax and H6ax. Therefore we expected two observable NOEs in the cis-configuration but only one NOE in the trans (Fig. 3). Experimentally, as shown in Fig. 4, for 7f, the signal at 4.47 ppm shows NOE with the signal at 3.58 ppm, the signal at 2.74 ppm shows NOE with the signal at 2.33 ppm, therefore this correspond to the cis-configuration. For 8f, the signal at 3.82 ppm shows NOE with the signal at 3.47 ppm, and therefore this correspond to the trans-configuration.
|
Download:
|
| Fig. 2.The 1H NMR spectra of compound 7f, 8f and 9. | |
|
Download:
|
| Fig. 3.NOE in cis- and trans-configuration. | |

|
Download:
|
| Fig. 4.NOESY spectrum of compound 7f and 8f. | |
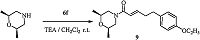
Finally, in order to verify this deduction, we further chose commercial available cis-2, 6-dimethylmorpholine reacted with (E)-5-(4-ethoxyphenyl)pent-2-enoyl chloride (6f) to obtain compound 9 (Scheme 2) with cis-configuration. As a result, the 1H NMR spectrum of 9 coincided exactly with the 1H NMR spectrum of compound 7f (Fig. 4). Therefore 7f should be the isomer with cisconfiguration. This result is same with the previous deduction. On the basis of the above results, we can identify both isomers of all the other target compounds.
|
Download:
|
| Scheme 2.Synthesis of compound 9. | |
The target compounds were evaluated for fungicidal activities against six plant pathogenic fungi (Valsa mali, Pythium aphanidermatum, Rhizoctorzia solani, Alternaria solani, Bipolaris maydis and Sclerotinia sclerotiorum) at the concentration of 50 mg/L according to the method reported in the literature [14]. Difenoconazole, a commercial fungicide, was used as positive control. The results were summarized in Table 1. Data in Table 1 indicated that these compounds showed fungicidal activities at different degree. Generally, these compounds exhibited better activities against V. mali than the other tested fungi. Among them, the inhibitory rates of 7c and 7d against V. mali reached 76.8% and 90.7%, respectively, obviously superior to that of the lead compound (Ⅰ) (45.1%). This result indicated that an electron-withdrawing substituent is more desirable than an electron-donating substituent to generate a compound with better fungicidal activity. What is more, amongst the analogs containing a halogen substituent on the benzene ring, the electronegativity of the halogen had a markedly influence on the fungicidal activities. The compounds containing a halogen with weaker electronegativity showed better activities against most of the tested fungi than those compounds containing a halogen with bigger electronegativity. For example, the order of the fungicidal activities of compounds 7b (with a fluorine substituent), 7c (with a chlorine substituent), and7d(with a brominesubstituent) could be placed as following: 7d > 7c > 7b. Also, compounds 8b, 8c, 8d, hadthe similar results.Andinterestingly, the configurationof the 2, 6-dimethylmorpholine moiety of target compounds had a remarkable effect on the fungicidal activities of these analogs. As awhole, the fungicidal activities against almost all the testedfungi of the isomer with cis-2, 6-dimethylmorpholine were better than the trans-isomer, especially against V. mali, P. aphanidermatum, R. solani and A. solani. Moreover, the activities of compounds 7g and 8g indicated that the introduction of thiophene group is unfavorable to the fungicidal activity.
|
|
Table 1 Fungicidal activities of the target compoundsa. |
In addition, the EC50 value of comparatively potent compounds 7c, 7d, 8c, and 8d against V. mali were further tested (Table 2). Compound 7d, with a 4-bromine-substituted benzyl group and cis- 2, 6-dimethylmorpholine moiety, exhibited the highest activity with an EC50 of 23.87 mmol/L, superior to compound Ⅰ (40.34 mmol/L), whereas, the EC50 of compounds 7c, 8c, and 8d are 43.56, 66.29, and 42.79 mmol/L, respectively. Same with the previous results, the fungicidal activities of compounds with cis- 2, 6-dimethylmorpholine moiety were better than the compounds with trans-2, 6-dimethylmorpholine moiety. For example, the EC50 of 7c was lower than 8c, and the EC50 of 7d was lower than 8d.
|
|
Table 2 The EC50 value of compounds 7c, 8c, 7d and 8d against Valsa mali. |
In summary, a series of novel daphneolone analogs containing 2, 6-dimethylmorpholine moiety were designed and synthesized on the basis of natural product 1, 5-diphenyl-2-penten-1-one (Ⅰ) from S. chamaejasme L. The two isomers of target compounds were separated and identified. All the compounds were evaluated for their fungicidal activities against six plant pathogenic fungi.
Bioassay results indicated that some of the target compounds exhibited good fungicidal activities against tested fungi at the concentration of 50 mg/L. Compound 7d, with a 4-brominesubstituted benzyl group and cis-2, 6-dimethylmorpholine moiety, showed the best fungicidal activity against V. mali with 90.7% inhibition rate at the concentration of 50 mg/L and EC50 value of 23.87 mmol/L. Initial structure-activity relationship revealed that, the configuration of target compounds had a remarkable effect on the fungicidal activities of these analogs. Between two isomers of the target compounds, the fungicidal activities of the isomer with cis-2, 6-dimethylmorpholine were better than the trans-isomer. Further studies on the structural optimization are in progress in our laboratory.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 21272266) and the National High Technology Research and Development Program of China (No. 2011AA10A202).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.01. 045.
| [1] | M. Farag, M.S. Mohammed, I. Foud, et al., The role of natural products in drug discovery and development, WJPR 4(2015) 22-33. |
| [2] | D.J. Newman, G.M. Cragg, Natural products as sources of new drugs over the 30 years from 1981 to 2010, J. Nat. Prod. 75(2012) 311-335. |
| [3] | P. Gao, T.P. Hou, R. Gao, Q. Cui, S.G. Liu, Activity of the botanical aphicides 1,5-diphenyl-1-pentanone and 1,5-diphenyl-2-penten-1-one on two species of Aphididae, Pest Manage. Sci. 57(2001) 307-310. |
| [4] | T.P. Hou, Q. Cui, S.H. Chen, N.T. Hou, S.G. Liu, New compounds against aphides from Stellera chamaejasme L, Chin. J. Org. Chem. 22(2002) 67. |
| [5] | P. Gao, Y.P. Liu, S.G. Liu, Effects of dp-B on ATPase activity of insect plasma membrane, Pestic. Biochem. Physiol. 80(2004) 157-162. |
| [6] | T. Lu, L. Chen, T.P. Hou, et al., Synthesis and structure-activity study of botanical aphicides 1,5-diphenyl-1-pentanone analogues, Pestic. Biochem. Phys. 89(2007) 60-64. |
| [7] | L. Chen, X.L. Wang, T. Lu, et al., Lead optimization and insecticidal activity of analogues of daphneolone isolated from Stellera chamaejasme L, Pest. Manage. Sci. 63(2007) 928-934. |
| [8] | H. Jin, Y.C. Geng, Z.Y. Yu, et al., Lead optimization and anti-plant pathogenic fungi activities of daphneolone analogues from Stellera chamaejasme L, Pestic. Biochem. Phys. 93(2009) 133-137. |
| [9] | S.X. Yang, Design, Synthesis and Bioactivity of Natural Product Daphneolone Analogues, China Agricultural University, Beijing, 2012, Ph.D. Dissertation. |
| [10] | R.L. Xie, Structure Modification and Bioactivity of Natural Active Compound Daphneolone, China Agricultural University, Beijing, 2014, Ph.D. Dissertation. |
| [11] | S.X. Yang, T.N. Kang, C.H. Rui, X.L. Yang, et al., Design, synthesis, and insecticidal activity of 1,5-diphenyl-1-pentanone analogues, Chin. J. Chem. 29(2011) 2394. |
| [12] | R.L. Xie, Y. Song, X.L. Yang, et al., Synthesis and fungicidal activity of novel daphneolone analogues, Chem. J. Chin. Univ. 35(2014) 1451. |
| [13] | C.M. Duarte, H. Verli, et al., New optimized piperamide analogues with potent in vivo hypotensive properties, Eur. J. Pharm. Sci. 23(2004) 363-369. |
| [14] | N.C. Chen, Bioassay of Pesticides, Beijing Agricultural University Press, Beijing, 1991, pp. 161-162. |
 2016, Vol.27
2016, Vol.27