Noninvasive bioluminescence imaging (BLI) has emerged as a routine technique in fields such as medical science, biochemistry and molecular biology [1, 2, 3]. Wide BLI applications have been discovered including real-time monitoring of gene expression [4], tumor growth [5] and protein-protein interactions [6]. The mechanism depends on an enzymatic oxidation reaction involving an enzyme (luciferase), a substrate (luciferin) and molecular oxygen, resulting in the emission of light.
To avoid absorption and scattering by tissue in small-animal imaging experiments, the wavelength range of light emissions had better be the red to near-infrared (600-900 nm). Firefly luciferase (Fluc, 61 kDa) is the most commonly utilized BLI system due to its favorable emission spectrum (emission spectra in the range of 540-615 nm), stability, and biocompatibility of its bioluminogenic substrate D-luciferin [7]. Unfortunately, however, cofactors such as ATP and Mg2+ ion are required which potentially leading to the experimental complexity in bioanalysis. On the other hand, renilla luciferases (Rluc, 36 kDa) in conjunction with coelenterazine (CTZ) or its derivatives as the substrate only require molecular oxygen [8]. Moreover, Rluc has been successfully expressed in mammalian cells without any cytotoxicity. However, the Rluc/CTZ pair produces a blue-green light with are latively short wavelength (450-475 nm). To overcome such a limitation, much effort has been made either by modifications of the RLuc [9] or by synthesis of new red-shifted CTZ analogues [10]. To our knowledge, it seems to be difficult to achieve red-shifted variants of RLuc via molecular biology approaches. Hence, we focus on the modifications of CTZ analogues to find a bathochromic and stable substrate of Rluc.
Since the enzymatic recognitionmechanismof the Rluc systemis not yet fully understood [11], the design of novel valuable CTZ derivatives is challenging, and only few red-shifted substrates have been reported so far. One significant molecule, v-coelenterazine owning a more planar and rigid molecular structure with a vinylene bridge at the C-5 position, displays a remarkable red-shifted emission at 512 nm and high quantum yield with RLuc, however at the cost of synthesis and molecular stability [10]. As to other CTZ derivatives modified at the C-2 [12], C-5 [10], C-6 [13] and C-8 [14] positions of the imidazopyrazinone core, few show luminescence properties superior to those of native CTZ, mostly since their structural modifications prevent their enzymatic recognition. In previous work, the luminescence properties of many modified CTZ derivatives are measured in comparison with coelenterazine 400a (DeepBlueCTM, 1). Coelenterazine 400a, a commercially available CTZ derivative, produces a -400 nm emission peak upon Rlucmediated oxidation. It is used for bioluminescence resonance energy transfer (BRET) studies because it hasminimal interference with the emission of the GFP acceptor, which provides greater signal resolution [6]. Recently, a sulfur-containing CTZ derivative 3 (Fig. 1) was discovered with a 30 nm red-shift compared to coelenterazine 400a in bioluminescence [15]. At the same time, the relative quantumyield (RQY) is 3.6 compared to coelenterazine 400a, measured by mixing substrates and rough cytosolic extract prepared from cells expressing Rluc. However, in cells there are many unwanted substances prone to combine with CTZ derivatives and catalyze the oxidation reaction such as albumin and other intrinsic proteins besides Rluc, thus giving rise to background noise in bioluminescence assays [16].
|
Download:
|
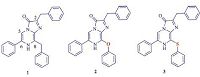
| Fig. 1.Structures of the coelenterazine analogs 1–3. | |
In this paper, we designed and synthesized a CTZ derivative that contains an oxygen atom in place of the native methylene group at C-8 position (1). We hypothesized that this replacement would also produce a red-shifted emission because of similar attributes of the oxygen and sulfuratom. To examine RQY, a new measurement method through an IVIS Kinetic equipped with a cooled chargecoupled- device (CCD) detector had been developed. Besides we employed commercially available purified Rluc enzyme in place of the rough cytosolic extract to decrease enzyme-independent luminescence (autoluminescence) of CTZ derivatives. To further evaluate the luminescence properties of CTZ derivatives, we performed the assay for luminescence activity in the live cell. All results showed that CTZ derivative 2 (Fig. 1) displayed a more significant red-shift (63 nm) in bioluminescence compared to coelenterazine 400a while it had lower quantum yield. In cell imaging photon emission from 3 was significantly higher compared to coelenterazine 400a (1.77 × 0.09; P ± 0.01), while compound 2 exhibited a 0.74 × 0.08 slightly lower luminescence signal. Therefore, replacement of the methylene group at the C-8 position with an O or S heteroatom is a new promising method to develop red-shifted CTZ analogs.
2. Experimental 2.1. Materials and apparatusAll reagents for organic synthesis were obtained from commercial suppliers and used without further purification. When necessary, organic solvents were routinely dried and/or distilled prior to use and stored over molecular sieves under argon. Millipore water was used to prepare all aqueous solutions. 1H NMR and 13C NMR were recorded on Bruker AV-300 or AV-600 spectrometers at the College of Chemistry NMR Facility, Shandong University. All chemical shifts are reported in the standard d notation of parts per million using the peaks of residual proton and carbon signals of the solvent as internal references. Mass spectra were recorded in ESI+ mode (70 eV) in Drug Analysis Center at Shandong University. ESI-HRMS was performed on a Waters SYNAPT G2-Si. The purity of CTZ analogues was confirmed by analytical reverse-phased HPLC (Agilent, 1260 Infinity) on Phenomenex C-18 column (250 × 4.6 mm). Melting points were determined on a Mel-Temp apparatus and were not corrected. Luminescence spectra were recorded using an F-2500 FL Spectrophotometer. The light outputs were determined with an IVIS Kinetic (Caliper Life Sciences, USA) equipped with a cooled CCD camera. All the experiments were carried out at room temperature unless otherwise specified.
2.2. Synthesis of CTZ derivatives 1-3The synthesis of compound 1-3 was shown in Schemes S1 and S2 in Supporting information. The details for preparation and NMR and HRMS spectra of these compounds were also presented in Supporting information.
2.3. Luminescence spectral analysisSynthesized CTZ derivatives were dissolved in ethanol at a concentration of 1 μg/mL for use as stock solutions, stored at-20 ℃ or lower temperature (for long-time storage), and diluted in 50mmol/L Tris-HCl buffer pH 7.4 (without calcium and magnesium) immediately prior to use. Enzyme assays were conducted using commercially available purified Rluc enzyme (Ray Biotech) at a concentration of 28 nmol/L in 1 mL PBS (pH 7.4) stored at -80 ℃.
Luminescence spectra were recorded using an F-2500 FL Spectrophotometer with the excitation lamp turned off and the emission shutter open at a scanning speed of 3000 nm/min. All the spectra were measured at room temperature. The in vitroin bioluminescence spectra were measured in 50 mmol/L Tris-HCl buffer pH 7.4 containing Rluc protein at a final concentration of 15 nmol/L and initiated by the injection of CTZ derivatives buffer solution at a final concentration of 50 mmol/L. Chemiluminescence spectra were traced by mixing 200 mL of CTZ derivatives buffer solution at a final concentration of 50 mmol/L with 800 mL of dimethylsulfoxide (DMSO). The response time is 2.0 s, and all spectra were not corrected for luminescence decay at spectral scanning. The luminescence spectra are the result of three independent measurements, each one measured in triplicate.
2.4. Relative quantum yield (RQY) and kinetics of in vitroin luminescenceThe RQY study and kinetic analysis were performed using an IVIS Kinetic (Caliper Life Sciences, USA) which consisted of a cooled charge-coupled device (CCD) camera mounted on a light-tight specimen chamber (dark box), a camera controller, a camera cooling system, and controlled using a computer. The data are represented as pseudocolor images (in photons/s/cm2/scr) of light intensity (blue—least intense, red—most intense) superimposed over the grayscale reference images. Circular specified regions of interest (ROIs) were drawnon the areas, and the light output were quantified as the total number of photons emitted per second using Living Image software.
To determine the appropriate unsaturatedamount of substrate, 10 mL of CTZ derivatives 1-3 between 1 and 100 mmol/L and 90 mL of either DMSO (chemiluminescence) or Rluc enzyme at a final concentration of 15 nmol/L (bioluminescence)were used. The RQY and reaction kinetics was determined by mixing 10 mL of CTZ derivatives (final concentration of 5 mmol/L) with 90 mL of either DMSO or Rluc enzyme (15 nmol/L) onto wells of 96-well black plates to prevent light reflection from well to well. Luminescent signals were measured immediately after mixing and monitored over a period of 25-30 min (luminescence had almost decayed to near-background levels) using the IVIS. Light output was recorded every 5 min with an exposure time of 30 s for chemiluminescence and every 1 minwith an exposuretime of 5 s in the first 15 min for bioluminescence. The collected data was analyzed by employing the Prism5.0 GraphPad software to compute the total light output. As a corresponding blank control, Tris-HCl buffer was added instead of CTZ derivatives solution under the same conditions. All assays were performed in triplicate.
2.5. Assay for luminescence activity in live cellES-2 cells (human ovarian cancers cell line) expressing Rluc were supplied by BioDiagnosis. The ES-2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; high glucose with L-glutamine; Gibco) containing 10% fetal bovine serum (FBS) and 0.5 μg/mL puromycin at 37 ℃ in a humidified atmosphere in a 5% CO2 incubator. CTZ derivatives was dissolved in ethanol to make a 1 mmol/L stock solution, and diluted with Tris-HCl buffer to gradient concentration (5, 10, 20, 40, 60, 80, 100 mmol/L).
The ES-2-Rluc cells were grown in black 96-well plates (4 × 104 cells per well). After a 24-h incubation period, the medium was removed. Then cells were washed with Tris-HCl buffer twice and treated with 100 mL of a series of concentrations of CTZ derivatives solutions (ranging from 0 to 100 mmol/L). Bioluminescent signals were then immediately determined using the IVIS. Light output was recorded every 1 min with an exposure time of 30 s until luminescence almost decayed to near-background levels. Luminescent signal (photons per second) for each well was measured and plotted as average values. All experiments were performed in triplicate.
3. Results and discussion 3.1. Luminescence spectraThe luminescence spectra of CTZ derivatives 1, 2 and 3 were determined using dimethylsulfoxide (DMSO) for chemiluminescence and Rluc enzyme for bioluminescence. As shown in Fig. 2a and Table S1 in Supporting information, sulfur-containing CTZ derivative 3 produced a 42 nm red-shifted spectrum measured in neutral DMSO relative to coelenterazine 400a (compound 1). However, oxygen-containing CTZ derivative 2 displayed a 10 nm blue-shift in chemiluminescence. Significantly, the sulfur and oxygen heteroatom replacement of the methylene bridge in coelenterazine 400a both lead to a bathochromic shift in the bioluminescent reaction with Rluc. The bioluminescence spectra exhibited a 36 nm red-shift for 3 and more significant red-shift (63 nm) for 2 compared to coelenterazine 400a (Fig. 2b). As a result, the differences between the emission peaks were generally attributed to an effective extension of π-electron conjugation, electronegativity, or H-bonds within the active site of oxygen and sulfur heteroatom at the C-8 position [17].

|
Download:
|
| Fig. 2.(a) Chemiluminescence emission spectra of coelenterazine derivatives; (b) Bioluminescence emission spectra of coelenterazine derivatives. | |
The quantum yield (QY) in bio/chemiluminescence is defined as the efficiency of the production of a photon from a single reactant molecule and is a key quantity to characterize the reaction [18]. In real experiments, QY is achieved by dividing the absolute number of all emitted photons by the number of substrate molecules consumed. However, because of different system manufacture and measuring conditions, it is difficult to gain a standard value of QY and it need not to for the purpose of this paper. Hence, we evaluated the luminescence properties by another related parameter, the relative quantum yield (RQY).
In this paper, we developed a new measurement method through an IVIS Kinetic equipped with a cooled charge-coupleddevice (CCD) detector as a sensitive photodetector to examine QY or RQY. The CCD detector has prominent advantages over traditional photomultiplier tubes (PMTs) [18], such as high quantum efficiency, low direct current noises, and parallel multichannel photo-detection, which are very appropriate for measuring efficiently.
Before acquiring RQY, we performed the kinetic analysis of in vitroin luminescence reactions and plotted kinetic profile of bio/ chemiluminescence presented as Fig. 3 and Fig. S1 in Supporting information. In coordinates, the vertical axis expresses the absolute number of photons per second, and the horizontal axis shows the reaction time, thus the area under the curve (AUC) meaning the absolute total number of luminescence photons. The absolute total number of photons divided by the number of reacted molecules gives QY. As a result, we also obtain RQY (relative to coelenterazine 400a).

|
Download:
|
| Fig. 3.Comparison of the chemiluminescence generated by the three CTZ derivatives in DMSO. (a) Dose-responsiveness of chemiluminescenceimmediately measured after the addition of CTZ derivatives ranging from 1 mmol/L to 100 mmol/L using the IVIS; (b) Representative chemiluminescence images shown after the addition of CTZ derivatives in 96-well plates; (c) Time course of light output following addition of 5 mmol/L substrate using the IVIS; (d) The reaction kinetics of different CTZ derivatives in chemiluminescence. All values are shown as mean ± SD (n = 3). | |
To determine the appropriate amount of substrate and discover an optimized condition tomeasureRQY, weset up the concentration of CTZ derivatives 1-3 ranging from 1 mmol/L to 100 mmol/L. As a result, the concentration at 5 mmol/L is suitable for both chemiluminescence and bioluminescence according to Fig. 3. First, RQY of CTZ derivatives 1-3 for chemiluminescence were measured, and it was 1.81 to compound 3, which is in good agreement with previously reported value [15]. While RQY was 0.77 to compound 2 inferior to coelenterazine 400a, the luminescence intensity of 2 was higher than coelenterazine 400a during the first 5 min. In the bioluminescence reaction, compounds 2 and 3 showed lower quantum yield (Table S1). Nevertheless, compound 3 owned a relative lower saturated concentration, indicating that it had a higher affinity to native renilla luciferase (Rluc). Meanwhile, 2 and 3 may show better bioluminescence intensities using Rluc variants such as the widely known Rluc 8 and Rluc 8.6 variants [9, 19] than coelenterazine 400a, which needed further study. Therefore, 2 and 3 are promising bright red-shifted CTZ derivatives.
Measurements of the chemiluminescence and bioluminescence emission time profiles revealed that all three CTZ derivatives show the typical flash-type luminescence kinetics [20]. For 1, an immediate burst to peak emission rate followed by steady decay and for 2 and 3, a same immediate burst to peak emission rate but followed by a relatively dramatic decay with shorter half-life times in both chemiluminescence and bioluminescence reactions, likely due to an intrinsic change in the electronic properties of the excited states of themolecule, or the different binding affinity of CTZ to Rluc.
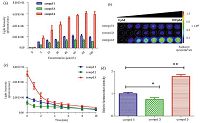
3.3. Luminescence activity in live cellNext, we performed the assay for luminescence activity in live ES-2 cells expressing Rluc. The total photons of the first 10min (i.e. AUC) were used to measuring luminescence signal intensity. Interestingly, photon emission from 3 was significantly higher compared with 1 (1.77 × 0.09; P ± 0.01). At the same time, 2 exhibited a 0.74 × 0.08 lower luminescence signal compared with 1 (Fig. 4). Considering low quantum yield in the bioluminescence reaction and highquantumyieldin the chemiluminescence reactionfor compound3, we think 3 or other hydrophobic CTZ derivativesmay produce enzymeindependent luminescence (autoluminescence) in complex intracellular environment after permeating through the cell membrane, for example albumin can catalyze CTZ chemiluminescence consistentwith a monooxygenase [16]. Thus, a higher luminescence signal was recorded compared with a pure bioluminescence.
|
Download:
|
| Fig. 4.Comparison of the luminescence generated by the three CTZ derivatives in ES-2 cells expressing Rluc. (a) Dose-responsiveness of luminescence immediately measured after the addition of CTZ derivatives ranging from 5 to 100 mmol/L; (b) Representative luminescence images shown after the addition of CTZ derivatives in 96-well plates; (c) Time course of light output following addition of 40 mmol/L substrate; (d) Photon flux comparison, compound 2 (* P ≤ 0.05)showing the significantly lower photon emission compared with coelenterazine 400a and compound 3 (** P ≤ 0.01) showing the significantly higher photon emission compared with coelenterazine 400a. All values are shown as mean ± SD (n = 3). | |
In conclusion, we have described an oxygen-containing CTZ derivative 2 and it exhibited a more significant red-shifted (63 nm) bioluminescence signal maximum relative to coelenterazine 400a, while it had lower quantumyield. The results of this study appear to support our hypothesis that the introduction of oxygen heteroatom at the C-8 position could also produce a bathochromic effect.While a possible mechanism of the CTZ luminescence reaction has been proposed (Fig. S2 in Supporting information), the enzymatic recognition mechanism of the Rluc system is not yet fully understood.Wemay surmise that it is due to an efficient extension of π-electron conjugation, electronegativity, orH-bonds of oxygen at the C-8 position. We also developed a new measurement method through an IVIS Kinetic equipped with a CCD to examine RQY. The RQYof CTZ derivative 3 for chemiluminescence is in good agreement with previously reported value. To get a genuine and correct RQY in bioluminescence, we employed commercially available purified Rluc enzyme in place of the rough cytosolic extract to avoid enzymeindependent luminescence. Compounds 2 and 3 showed lower quantumyield that is quite different frompreviously reported value. However, we discovered compound 3 had a higher affinity to Rluc than coelenterazine 400a. In cell imaging, compound 3 also displayed a higher luminescence signal. In a word, compounds 2 and 3 are promising bright red-shifted CTZ derivatives that provide a novel approach to improve the luminescence properties of CTZ analogs. More significantly, it is beneficial to understand the underlying mechanisms of Rluc bioluminescence upon reacting with coelenterazine.
AcknowledgmentsThis work was supported by grants from the National Program on Key Basic Research Project (No. 2013CB734000), the National Natural Science Foundation of China (No. 81370085), the Major Project of Science and Technology of Shandong Province (No. 2015ZDJS04001) and the Fundamental Research Funds of Shandong University (No. 2014JC008).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.02.011.
| [1] | X. Xu, M. Soutto, Q. Xie, et al., Imaging protein interactions with bioluminescence resonance energy transfer (BRET) in plant and mammalian cells and tissues, Proc. Natl. Acad. Sci. U.S.A. 104(2007) 10264-10269. |
| [2] | T. Kimura, K. Hiraoka, N. Kasahara, C.R. Logg, Optimization of enzyme-substrate pairing for bioluminescence imaging of gene transfer using Renilla and Gaussia luciferases, J. Gene Med. 12(2010) 528-537. |
| [3] | T.C. Zhang, L.P. Du, M.Y. Li, BioLeT:a new design strategy for functional bioluminogenic probes, Chin. Chem. Lett. 26(2015) 919-921. |
| [4] | M.P. Hall, J. Unch, B.F. Binkowski, et al., Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate, ACS Chem. Biol. 7(2012) 1848-1857. |
| [5] | T. Suzuki, C. Kondo, T. Kanamori, S. Inouye, Video rate bioluminescence imaging of secretory proteins in living cells:localization, secretory frequency, and quantification, Anal. Chem. 415(2011) 182-189. |
| [6] | K.D.G. Pfleger, J.R. Dromey, M.B. Dalrymple, et al., Extended bioluminescence resonance energy transfer (eBRET) for monitoring prolonged protein-protein interactions in live cells, Cell. Signal. 18(2006) 1664-1670. |
| [7] | W.X. Wu, J. Li, L.Z. Chen, et al., Bioluminescent probe for hydrogen peroxide imaging in vitro and in vivo, Anal. Chem. 86(2014) 9800-9806. |
| [8] | J. Levi, A. De, Z. Cheng, S.S. Gambhir, Bisdeoxycoelenterazine derivatives for improvement of bioluminescence resonance energy transfer assays, J. Am. Chem. Soc. 129(2007) 11900-11901. |
| [9] | A.M. Loening, A.M. Wu, S.S. Gambhir, Red-shifted Renilla reniformis luciferase variants for imaging in living subjects, Nat. Methods 4(2007) 641-643. |
| [10] | T. Hosoya, R. Iimori, S. Yoshida, et al., Concise synthesis of v-coelenterazines, Org. Lett. 17(2015) 3888-3891. |
| [11] | H. Isobe, S. Yamanaka, S. Kuramitsu, K. Yamaguchi, Regulation mechanism of spin-orbit coupling in charge-transfer-induced luminescence of imidazopyrazinone derivatives, J. Am. Chem. Soc. 130(2008) 132-149. |
| [12] | S. Inouye, Y. Sahara-Miura, J. Sato, et al., Expression, purification and luminescence properties of coelenterazine-utilizing luciferases from Renilla, Oplophorus and Gaussia:comparison of substrate specificity for C2-modified coelenterazines, Protein Expression Purif. 88(2013) 150-156. |
| [13] | R. Nishihara, H. Suzuki, E. Hoshino, et al., Bioluminescent coelenterazine derivatives with imidazopyrazinone C-6 extended substitution, Chem. Commun. 51(2015) 391-394. |
| [14] | C. Wu, H. Nakamura, A. Murai, O. Shimomura, Chemi- and bioluminescence of coelenterazine analogues with a conjugated group at the C-8 position, Tetrahedron Lett. 42(2001) 2997-3000. |
| [15] | G. Giuliani, P. Molinari, G. Ferretti, et al., New red-shifted coelenterazine analogues with an extended electronic conjugation, Tetrahedron Lett. 53(2012) 5114-5118. |
| [16] | N. Vassel, C.D. Cox, R. Naseem, et al., Enzymatic activity of albumin shown by coelenterazine chemiluminescence, Luminescence 27(2012) 234-241. |
| [17] | G.A. Stepanyuk, Z.J. Liu, S.S. Markova, et al., Crystal structure of coelenterazinebinding protein from Renilla muelleri at 1.7 A:why it is not a calcium-regulated photoprotein, Photochem. Photobiol. Sci. 7(2008) 442-447. |
| [18] | Y. Ando, K. Niwa, N. Yamada, et al., Development of a quantitative bio/chemiluminescence spectrometer determining quantum yields:re-examination of the aqueous luminol chemiluminescence standard, Photochem. Photobiol. 83(2007) 1205-1210. |
| [19] | S.V. Markova, E.S. Vysotski, Coelenterazine-dependent luciferases, Biochemistry (Moscow) 80(2015) 714-732. |
| [20] | K. Teranishi, Luminescence of imidazo[1,2-a]pyrazin-3(7H)-one compounds, Bioorg. Chem. 35(2007) 82-111. |
 2016, Vol.27
2016, Vol.27 


