b The USTC-Anhui Tobacco Joint Laboratory of Tobacco Chemistry, Research Center of Tobacco and Health, University of Science and Technology of China, Hefei 230052, China
Tetracycline (TC), a kind of broad-spectrum antibiotics, had been widely used to treat bacterial infections in many fields during last century [1, 2]. However, many undesirable adverse effects including chronic toxicity, hepatotoxicity, and impact on tooth development have been found gradually [3]. Nowadays, the pollution of TC in surface and ground water has been proved to be a serious environmental concern due to the improper use and the relatively high solubility of TC in water (∼170 mg/100 g water) [4, 5]. Therefore, the development on the effective removal of TC from the polluted water has become an urgent task. Until now, various materials including montmorillonite [6, 7, 8], carbon nanotube [9], and graphene oxide (GO) [10, 11, 12] have been reported as the adsorbents for TC. Among them, graphene oxide (GO) has attracted considerable attention owing to the plenty of oxygen functional groups, intercalating and ion exchange property [13, 14], and the strong affinity of GO to molecules containing phenyl caused by the strong electrostatic attraction force and π-π interaction [15]. Many attempts have been made to fabricate GObased adsorbents with different morphologies, such as sheet-like GO [16] and three-dimensional macroporous GO [17, 18], to increase the amount of the active sites on GO adsorbents so as to obtain an improved adsorption performance. For example, Gao et al. [17] used a unidirectional freeze-drying method to prepare GO aerogels with continuous pore structure, which have an equilibrium adsorption capacity for Cu2+ in the aqueous solution as high as 19.1 mg g-1 and a fast adsorption rate. However, pure macroporous structured GO generally exhibits poor mechanical property. Meanwhile, the agglomeration of GO sheets during the fabrication process also leads to the loss of the adsorption capacity. A feasible way to solve the above mentioned problems is to make GO distribute homogeneously into a strong polymer matrix to form macroporous polymer/GO composites.
Pickering emulsion droplets can be used as good macropore templates [19]. Besides the common inorganic or polymeric particulate stabilizers, GO has also been proved to be able to act as the stabilizer for a Pickering emulsion since the GO sheets have the hydrophilic edges due to the oxygenated groups and the hydrophobic surface due to the graphene-like aromatic structure [20]. It is noted that the hydroxyl groups on the surface of the particulate stabilizers will enhance the interaction between the agglomerated Pickering emulsion droplets through the hydrogen bond interaction, leading to the formation of a self-stand monolith material with a matrix composed of the stabilizer particles [[21],[22],[23]]. It has been reported that abundant oxygenated groups including hydroxyl groups exist on the GO sheets [24]. However, there are few reports referring to prepare macroporous GO-based monolith from a Pickering emulsion system stabilized by GO sheets, taking advantage of the hydrogen bond interaction between the oxygenated groups on GO sheets.
Herein, we employed GO coated polystyrene microspheres (PS@GO) as the stabilizers to form an octane/water Pickering emulsion. During the evaporation process of water and octane, the Pickering emulsion droplets aggregated together, and combined with each other through the hydrogen bond interaction between the stabilizers. Finally, a three-dimensional macroporous PS/GO composite monolith was formed after all of the liquid components were evaporated. The macroporous PS/GO monolith showed an excellent adsorption ability for TC in aqueous solution with a maximum adsorption capacity of 197.8 mg g-1. At the same time, the macroporous PS/GO composite monolith has an excellent mechanical strength, which can bear a load of 2500 times its own weight. This work provides a new way to prepare high-strength and high-efficiency macroporous GO-based monolith adsorbents for the pollutants such as organic dyes, antibiotics, and heavy metal ions.
2. Experimental 2.1. MaterialsAnalytical grade styrene (St) provided by Shanghai Chemical Reagents Co., Ltd. was purified by vacuum distillation before use. Analytical reagents including 2, 20-azobis(isobutyramidine) dihydrochloride (AIBA) (99%), phosphorus pentoxide (P2O5), sodium hydroxide (NaOH), potassium persulfate (KPS), octane, H2SO4 (98%), HCl (37%), KMnO4, and H2O2 (30%) were all purchased from Shanghai Chemical Reagents Co., Ltd., and used as received. Polyvinylpyrrolidone (PVP) was purchased from Aladdin Reagents (Shanghai) Co., Ltd. Tetracycline hydrochloride (98%) was provided by J&K Scientific Ltd. Graphite flakes (100 mesh) were supplied by Beijing Jixing Sheng’an Industry & Trade Co., Ltd. Deionized water was used in all the experiments.
2.2. Preparation of macroporous PS/GO composite monolithGO and PSmicrospheres were prepared by amodified Hummer’s methodandemulsionpolymerization, respectively inour laboratory according to the literatures [25, 26]. The detailed preparation processes were described in the Supporting information (Part Ⅰ and Ⅱ). The as-preparedGOwas dispersed into 10mL ofwater under ultrasonification at a concentration of 4 mg mL-1.After the pHof the dispersion was adjusted to 2 with 1 mol L-1 HCl, PS microspheres were dispersed into the dispersion ultrasonically.Theweight ratio of PS microspheres to GO was controlled to 1:2. Then, octane (5 mL) was added into the PS/GO dispersion to form a Pickering emulsion using a high-shear dispersion homogenizer (FJ200-SH, 15, 000 rpm, 30 s). Afterwards, the emulsion was dried at 40 ℃ for 7 days to obtain the porous structured monolith. As a comparison, pure macroporous GO monolith was prepared according to the above same fabrication procedure without the addition of PS microspheres.
2.3. Adsorption ability of PS/GO composite monolith for TCThe adsorption kinetics of TC on PS/GO absorbent in aqueous solutions was investigated as follows: 10 mg of the sample adsorbent was dispersed into 50 mL of the aqueous solution of TC with a concentration of 29.3 mg L-1. The suspension was shaken in a WHY-2 shaker (150 rpm) at 25 ℃. 3 mL of the suspension was sampled at certain time intervals, and centrifuged to collect the supernatant. The UV-vis spectrum of the supernatant was recorded. The concentration of TC was determined from the absorption intensity at 357 nm. As a control, the adsorption ability of pure GO was measured under the same condition.
The adsorption capacity of PS/GO absorbent for TC in aqueous solution at different pH was also investigated. The pH of the suspension was adjusted to be 2, 4, 6, 8, and 10 using 1 mol L-1 HCl or 1 mol L-1 NaOH. 10 mg of the adsorbent and 50 mL of TC solution (29.3 mg L-1) with different pH were mixed and shaken at 25 ℃ for 12 h to reach the adsorption equilibrium. Then 3 mL of the suspension was sampled and centrifuged to collect the supernatant. The UV-vis spectrum of the supernatant was recorded to measure the concentration of TC from the absorption intensity at 357 nm.
To obtain the adsorption isotherm of PS/GO monolith at pH 6, 50 mL of TC solution with a series of concentrations (16.4- 132.7 mg L-1) were adopted to the adsorption experiments. The equilibrium adsorption capacity for TC (qe, mg g-1) was calculated according to the following equation:

The morphologies of the samples were investigated by fieldemission scanning electron microscopy (FESEM, JEOL JSM-6700F, 5 kV) and transmission electron microscopy (TEM, H-7650, 100 kV). The optical microscopy of the Pickering emulsion was observed by Leica DM 1000. X-ray photoelectron spectroscopy (XPS) was conducted on Thermo ESCALAB 250 using monochromatic Al Kα radiation. The photos of the emulsions were taken by a digital camera. The zeta potentials of PS microspheres and GO in the aqueous solution at pH 2 were measured by dynamic light scattering analysis (DLS) on a Malvern Zetasizer NanoZS-2S90 instrument. The particle concentration in the sample solutions was 0.05 mg mL-1. UV-vis spectra of the aqueous solution of TC and GO were obtained using a UV-2600. Thermo-gravimetric analysis (TGA) was operated on TGA Q5000IR in air at a heating rate of 10 ℃ min-1. The density of the PS/GO composite monolith was also measured by weighing method, as expressed by the following equation:

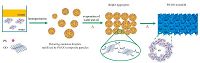
The fabrication process of a macroporous PS/GO composite monolith is illustrated in Scheme 1. First, PS microspheres were dispersed into the aqueous solution of GO to form the PS/GO composite particles, which can be used as the Pickering emulsion stabilizer. Fig. 1 shows the morphologies of PS microspheres, GO sheets, and the PS/GO composite particles. The PS microspheres are uniform in size with an average diameter of 267 nm (Fig. 1A). The pure GO sheets appear wrinkled and have a lateral size of around 7-8 mm (Fig. 1B). GO has electron-rich π bonds, as shown in Fig. 2A. The UV-vis spectrum of the aqueous dispersion of GO exhibits a sharp absorption peak at around 230 nm and a shoulder peak at around 290 nm, which are assigned to π→π* transition of aromatic C-C bonds and n→π* transition of C=O bonds, respectively [27]. Meanwhile, XPS spectrum of GO is shown in Fig. 2B. The main peak at 284.6 eV originates from sp2 carbon atoms. The peaks centred at 287.2 eV and 289.1 eV are due to C-O and C=O, respectively [28], indicating the existence of the oxygenated groups on GO sheets. When the prepared GO was dispersed into an acidic solution (pH 2), the zeta potential of GO was -17.4 ± 6.6 mV. But the zeta potential of PS microspheres in the same acidic solution was +2.75 mV, which should be attributed to the positively charged end groups of the initiator AIBA [29]. Therefore, the PS microspheres would be adsorbed on the surface of GO sheets in the aqueous solution to form PS/GO composite particles through the electrostatic attraction interaction, as well as theπ-π interaction, and van der Waals’ force [29], as shown clearly in Fig. 1C.
|
Download:
|
| Scheme 1.Illustration of the preparation process of macroporous PS/GO composite monolith. | |

|
Download:
|
| Fig. 1.TEM image of PS microspheres (A), GO (B), and the PS/GO composite particles in the aqueous solution at pH 2 (C). The insets are the corresponding SEM images. | |

|
Download:
|
| Fig. 2.UV–vis spectrum of the aqueous dispersion of GO (A) and XPS spectrum of GO (B). | |
Fig. 3A1-A5 shows the optical microscopy images and the corresponding digital photos of the emulsions formed after the addition of octane in the aqueous dispersion containing the PS/GO composite particles at pH2. It can be seen that theemulsion is stable for over 72 h. The diameters of emulsion droplets range from 5 mm to 50 mm. It is also noted that a layer of transparent liquid phase can be observed at the bottom, but the volume and the colour of this liquid phase were not change as the store time went on. The lamination may be caused by the relatively low concentration of the PS/GO composite particle stabilizer in this system because when the concentration of GO was raised to 6 mg mL-1, the Pickering emulsion can be stored stably without any lamination for over 72 h, as shown in Fig. 3B1-B5. The good stability of the emulsion indicates that the formed PS/GO composite particles can act as good Pickering emulsion stabilizers. The reason why PS/GO composite particles possess this unique property should be related with the amphiphilicity of GO sheets. The planes of the carbon network and three kinds of oxygenated groups (hydroxyl, carboxyl, and epoxy groups) make GO sheets behave like functional surfactants [24] since they can reduce the interfacial energy between water and oil [20].

|
Download:
|
| Fig. 3.The optical microscopy images of the Pickering emulsion stabilized by PS/GO composite particles after being stored for different time. The concentration of GO is 4mgmL-1 for (A1)–(A5) and 6 mg mL-1 for (B1)–(B5). The insets are the appearances of the corresponding emulsions taken by a digital camera. | |
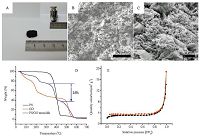
With the evaporation of water and octane, the Pickering emulsion droplets aggregated together, and finally a rigid PS/GO composite monolith material was successfully obtained, as shown in Fig. 4A. The density of the sample was measured to be 45.3 mg cm-3, which is much smaller than that of PS (1050 mg cm-3) and commercial GO powder (50-160 mg cm-3). 3). It means that the PS/GO composite monolith should possess massive pores. Fig. 4B gives the SEM image of the PS/GO composite monolith, which clearly proves the existence of continuous macroporous structure in it. The pore size has a distribution from 4 mm to 20mm, generally smaller than the Pickering emulsion droplets, indicating a volume shrinkage of the droplets during the drying process since the pores should be formed after the evaporation of octane according to Scheme 1. The magnified image of the wall of the pores is shown in Fig. 4C. It can be seen that the wall of the pores is composed of the GO sheets with PS microspheres wrapped in them. The TGA analysis was conducted to determine the composition of the PS/GO composite monolith, as shown in Fig. 4D. It is seen from the TGA curve of PS microspheres that the decomposition of PS microspheres occurs within the temperature range of 325-450 ℃. As for GO, the mass loss before 210 ℃ should be ascribed to the loss of water and impurities. The oxygen-containing groups decompose mainly before 350 ℃ [13]. When the temperature is above 488 ℃, a rapid mass loss of GO appears, indicating the decomposition of carbon skeleton. Thus, the weight loss of 35% between 325-450 ℃ on the TGA curve of PS/GO composite monolith should be attributed to the decomposition of PS component. The weight ratio of PS microspheres to GO should be calculated accordingly to be 1:1.86, which is very close to the feed ratio (1:2). The nitrogen adsorption-desorption isotherm of PS/GO composite monolith (Fig. 4E) is the typical type IV curve with nearly no hysteresis loop, according to BDDT classification [30]. A rapid increase of adsorption of nitrogen only occurs when P/P0 is above 0.9, implying the existence of macropores. This is in accord with the morphology displayed in Fig. 4B. The BET surface area of PS/GO composite monolith is 10.3 m2 g-1. It is found that the macroporous PS/GO composite monolith possesses a high strength. It will not be crushed when loaded with a weight of 100 g which is more than 2500 times over its own weight, as shown in the inset picture of Fig. 4A. The good mechanical performance should be attributed to two factors. First, the hydrogen-bond between the carboxyl and hydroxyl groups on the different GO sheets located at the emulsion droplet interfaces will be formed when the emulsion droplets contact with each other during the evaporation of water, as illustrated in Scheme 1. These hydrogen-bonds enhance the interactions between the GO sheets, resulting in the formation of continuous porous GO matrix (as seen in the Supporting information, Part Ⅲ Fig. S1A, macroporous pure GO material also can be prepared with the same method at the absence of PS microspheres). Second, the incorporation of PS microspheres enhances the strength of the GO matrix since the pure GO porous matrix is very brittle as seen in Fig. S1B.
|
Download:
|
| Fig. 4.The digital photo (A), SEM image (B) and (C), TGA curves of PS, GO and PS/GO monolith (D), and N2 adsorption-desorption isotherm (E) of PS/GO composite monolith. The inset photo in (A) shows that a weight of 100 g is put on the PS/GO composite monolith of 40 mg. | |
for TC The adsorption performance of the prepared macroporous PS/ GO composite monolith for TC in the aqueous solution was investigated using UV-vis spectroscopy. The working curve and the UV-vis spectra of the TC solution were presented in Fig. S2 in Supporting information. The PS/GO composite monolith exhibits the adsorption capacity of 92.75 mg g-1, which is 1.4 times that of pure GO (65.7 mg g-1). It indicates that the three-dimensional macroporous structure can provide much more active adsorption sites for TC molecules. Meanwhile, according to the molecular structure of TC (Fig. 5A), the pH of the solution will affect the protonation of -OH or amino groups so as to affect the interaction between TC and the adsorption medium. Thus, the dependence of the adsorption capacity of the macroporous PS/GO composite monolith on pH was first investigated, as shown in Fig. 5A. It is clearly seen that the adsorption capacity for TC in acidic solutions is generally much higher than that in basic solutions. It is reported that the protonation-deprotonation processes of tricarbonxyl methane group, phenolic diketone group, and dimethylamine groups will produce cationic species (pH < 3.3), zwitterionic species (3.3 < pH < 7.68), or anionic species (pH > 7.68) [9, 31], while GO has an overall negative surface charge at pH 2-12 [24]. It means that in a solutionwith a pH of above 7.68, both the surface of PS/GO composite monolith and TC molecules are negatively charged. The electro-repulsive force will counteract the π-π attraction interaction between GO and TC, resulting in the decrease of the adsorption capacity [9, 32]. When pH is less than 4, the TC molecule is positively charged. But the electronegativity of GO will also be weakened. Thus, the adsorption capacity will also be limited. When pH is between 4 and 6, the synergetic effect of electrostatic attraction and π-π attraction interaction between TC and GO reaches the maximum, so the adsorption capacity for TC does.

|
Download:
|
| Fig. 5.The adsorption capacity of macroporous PS/GO composite monolith for TC in an aqueous solution at different pH (A) (the inset is the molecular structure of TC); the kinetic adsorption amount of TC with time in an aqueous solution at pH 6 (B); the fitting plot of the data in (B) using the pseudo-first-order kinetic model (C) and the pseudo-second-order kinetic model (D). The initial concentration of TC was 29.3 mg L-1. | |
The adsorption kinetics of TC on macroporous PS/GO composite monolith in the aqueous solution at pH 6 had been studied as the initial concentration of TC (C0) was set as 29.3 mg L-1. The adsorbed amount of TC (qt) over time is plotted in Fig. 5B. It is seen that qt increases rapidly within the initial 50 min, then rises slowly in the following 200 min, and finally levels off. The equilibrium adsorption amount (qe (exp)) is 92.75 mg g-1. The data of Fig. 5B can be analysed with two classic adsorption kinetic models, i.e., pseudo-first-order kinetic model and pseudo-second-order kinetic model, which are expressed by the following Eqs. (3) and (4), respectively [33, 34]:
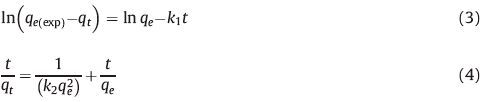
|
|
Table 1 Kinetic parameters for the adsorption of TC on macroporous PS/GO composite monolith (pH 6, 25 ℃). |
The adsorption isotherm of TC on macroporous PS/GO composite monolith was further studied. Fig. 6A shows the relationship between the adsorption amount and the concentration of TC in the test solutions when the adsorption equilibrium was reached. The data can be analysed by Langmuir and Freundlich isotherm models, respectively, as displayed in Fig. 6B and C. The Langmuir isotherm model is expressed by Eq. (5), which describes the homogeneous adsorption surface with all the adsorption sites having equal adsorption affinity [35, 36, 37]. The Freundlich isotherm model is expressed by Eq. (6), which assumes heterogeneity of adsorption surface [34, 36, 37].


|
Download:
|
| Fig. 6.The adsorption isotherm of TC at pH 6, 25 ℃ (A); the fitting plot of the data in (A) using the Langmuir isotherm model (B) and the Freundlich isotherm model (C). | |
|
|
Table 2 The thermodynamic adsorption parameters of TC calculated by Langmuir and Freundlich isotherm model at 25 ℃. |
The linear regression coefficient R2 for Langmuir isotherm model is as high as 0.9934, indicating that TC was homogeneously adsorbed on the whole surface of the macroporous PS/GO composite monolith. The calculated qmax from the Langmuir isotherm is 217.9 mg g-1, which is almost consistent with the experimental data of 197.9 mg g-1.
4. ConclusionIn summary, macroporous PS/GO composite monolith was fabricated using Pickering emulsion droplets as the soft template, which were stabilized by the in-situ formed PS/GO composite particles in water phase. The Pickering emulsion droplets agglomerated with the evaporation of water. At the same time, the hydrogen bonds generated by the carboxyl and hydroxyl groups on the different GO sheets located at the surface of the agglomerated Pickering emulsion droplets strengthened the interactions between the PS/GO composite particles, leading to the formation of the macroporous PS/GO composite matrix after the evaporation of both oil phase and water. The SEM images show that the obtained PS/GO monolith possesses macropores with diameters ranging from 4 mm to 20 mm. The incorporation of PS microspheres enhanced the strength of the macroporous monolith. The prepared macroporous PS/GO composite monolith had a high mechanical strength, which can bear a load of 2500 times its own weight. The adsorption ability of the macroporous PS/GO composite monolith for TC in the aqueous solution had been investigated. It was found that the monolith exhibited the maximum adsorption capacity in an aqueous solution at pH 4- 6. The maximum adsorption capacity at equilibrium can reach 197.9 mg g-1 at pH 6. The adsorption behaviour of TC on the macroporous PS/GO composite monolith fitted well with the Langmuir model and pseudo-second-order kinetic model. This preparation approach can be expanded to fabricate macroporous GO-based monolith absorbents with high strength and adsorption ability for organic pollutants.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (Nos. 51073146, 51103143, 51173175, 51473152, and 51573174), the Fundamental Research Funds for the Central Universities (Nos. WK2060200012 and WK3452016040601), and the Foundation of Anhui Key Laboratory of Tobacco Chemistry (China Tobacco Anhui Industrial Co., Ltd.) (No. 2014126).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2016.01.057.
| [1] | L. Chopra, M. Roberts, Tetracycline antibiotics:mode of action, applications, molecular biology, and epidemiology of bacterial resistance, Microbiol. Mol. Biol. Rev. 65(2001) 232-260. |
| [2] | Y.J. Feng, D. Zhong, H. Miao, X.M. Yang, Carbon dots derived from rose flowers for tetracycline sensing, Talanta 140(2015) 128-133. |
| [3] | Z. Cetecioglu, B. Ince, M. Gros, et al., Chronic impact of tetracycline on the biodegradation of an organic substrate mixture under anaerobic conditions, Water Res. 47(2013) 2959-2969. |
| [4] | V.M. D'Costa, C.E. King, L. Kalan, et al., Antibiotic resistance is ancient, Nature 477(2011) 457-461. |
| [5] | J. Beausse, Selected drugs in solid matrices:a review of environmental determination, occurrence and properties of principal substances, TrAC, Trends Anal. Chem. 23(2004) 753-761. |
| [6] | P.H. Chang, Z.H. Li, W.T. Jiang, C.Y. Kuo, J.S. Jean, Adsorption of tetracycline on montmorillonite:influence of solution pH, temperature, and ionic strength, Desalin. Water Treat. 55(2015) 1380-1392. |
| [7] | M.E. Parolo, M.J. Avena, G.R. Pettinari, M.T. Baschini, Influence of Ca2+ on tetracycline adsorption on montmorillonite, J. Colloid Interface Sci. 368(2012) 420-426. |
| [8] | H.Z. Xu, X.L. Qu, H. Li, C. Gu, D.Q. Zhu, Sorption of tetracycline to varying-sized montmorillonite fractions, J. Environ. Qual. 43(2014) 2079-2085. |
| [9] | F. Yu, J. Ma, S. Han, Adsorption of tetracycline from aqueous solutions onto multi-walled carbon nanotubes with different oxygen contents, Sci. Rep. 4(2014) 5326. |
| [10] | Y. Gao, Y. Li, L. Zhang, et al., Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide, J. Colloid Interface Sci. 368(2012) 540-546. |
| [11] | E.E. Ghadim, F. Manouchehri, G. Soleimani, et al., Adsorption properties of tetracycline onto graphene oxide:equilibrium, kinetic and thermodynamic studies, PLoS ONE 8(2013) e79254. |
| [12] | Y.X. Lin, S. Xu, J. Li, Fast and highly efficient tetracyclines removal from environmental waters by graphene oxide functionalized magnetic particles, Chem. Eng. J. 225(2013) 679-685. |
| [13] | S.H. Jiang, Z. Gui, C.L. Bao, et al., Preparation of functionalized graphene by simultaneous reduction and surface modification and its polymethyl methacrylate composites through latex technology and melt blending, Chem. Eng. J. 226(2013) 326-335. |
| [14] | D.R. Dreyer, A.D. Todd, C.W. Bielawski, Harnessing the chemistry of graphene oxide, Chem. Soc. Rev. 43(2014) 5288-5301. |
| [15] | S. Chowdhury, R. Balasubramanian, Recent advances in the use of graphenefamily nanoadsorbents for removal of toxic pollutants from wastewater, Adv. Colloid Interface Sci. 204(2014) 35-56. |
| [16] | D.W. Kim, L.G. Bach, S.S. Hong, C. Ark, K.T. Lim, A facile route towards the synthesis of Fe3O4/graphene oxide nanocomposites for environmental applications, Mol. Cryst. Liq. Cryst. 599(2014) 43-50. |
| [17] | X. Mi, G.B. Huang, W.S. Xie, et al., Preparation of graphene oxide aerogel and its adsorption for Cu2+ ions, Carbon 50(2012) 4856-4864. |
| [18] | Z.Q. Niu, L.L. Liu, L. Zhang, X.D. Chen, Porous graphene materials for water remediation, Small 10(2014) 3434-3441. |
| [19] | J.J. Blaker, K.Y. Lee, X.X. Li, A. Menner, A. Bismarck, Renewable nanocomposite polymer foams synthesized from Pickering emulsion templates, Green Chem. 11(2009) 1321-1326. |
| [20] | J.J. Shao, W. Lv, Q.H. Yang, Self-assembly of graphene oxide at interfaces, Adv. Mater. 26(2014) 5586-5612. |
| [21] | E. Garcia-Tunon, S. Barg, R. Bell, et al., Designing smart particles for the assembly of complex macroscopic structures, Angew. Chem. Int. Ed. 52(2013) 7805-7808. |
| [22] | C.H. Cao, M. Daly, B. Chen, et al., Strengthening in graphene oxide nanosheets:bridging the gap between interplanar and intraplanar fracture, Nano Lett. 15(2015) 6528-6534. |
| [23] | S.J. Wan, J.S. Peng, Y.C. Li, et al., Use of synergistic interactions to fabricate strong, tough, and conductive artificial nacre based on graphene oxide and chitosan, ACS Nano 9(2015) 9830-9836. |
| [24] | Y.Q. He, F. Wu, X.Y. Sun, R.Q. Li, et al., Factors that affect Pickering emulsions stabilized by graphene oxide, ACS Appl. Mater. Interfaces 5(2013) 4843-4855. |
| [25] | S.H. Che Man, S.C. Thickett, M.R. Whittaker, P.B. Zetterlund, Synthesis of polystyrene nanoparticles "armoured" with nanodimensional graphene oxide sheets by miniemulsion polymerization, J. Polym. Sci., A:Polym. Chem. 51(2013) 47-58. |
| [26] | G.G. Qi, Y.B. Wang, L. Estevez, et al., Facile and scalable synthesis of monodispersed spherical capsules with a mesoporous shell, Chem. Mater. 22(2010) 2693-2695. |
| [27] | B.W. Zhang, L.F. Li, Z.Q. Wang, S.Y. Xie, et al., Radiation induced reduction:an effective and clean route to synthesize functionalized graphene, J. Mater. Chem. 22(2012) 7775-7781. |
| [28] | E. Lee, J.Y. Hong, H. Kang, J. Jang, Synthesis of TiO2 nanorod-decorated graphene sheets and their highly efficient photocatalytic activities under visible-light irradiation, J. Hazard. Mater. 219-220(2012) 13-18. |
| [29] | Q.G. Shao, J. Tang, Y.X. Lin, F.F. Zhang, et al., Synthesis and characterization of graphene hollow spheres for application in supercapacitors, J. Mater. Chem. A 1(2013) 15423-15428. |
| [30] | S. Brunauer, L.S. Deming, W.E. Deming, E. Teller, On a theory of the van der Waals adsorption of gases, J. Am. Chem. Soc. 62(1940) 1723-1732. |
| [31] | L. Zhang, X.Y. Song, X.Y. Liu, et al., Studies on the removal of tetracycline by multiwalled carbon nanotubes, Chem. Eng. J. 178(2011) 26-33. |
| [32] | O.G. Apul, T. Karanfil, Adsorption of synthetic organic contaminants by carbon nanotubes:a critical review, Water Res. 68(2015) 34-55. |
| [33] | Y.Y. Xie, J. Wang, M.Z. Wang, X.W. Ge, Fabrication of fibrous amidoxime-functionalized mesoporous silica microsphere and its selectively adsorption property for Pb2+ in aqueous solution, J. Hazard. Mater. 297(2015) 66-73. |
| [34] | L.L. Fan, C.N. Luo, X.J. Li, et al., Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue, J. Hazard. Mater. 215-216(2012) 272-279. |
| [35] | S.L. Luo, X.L. Xu, G.Y. Zhou, et al., Amino siloxane oligomer-linked graphene oxide as an efficient adsorbent for removal of Pb(Ⅱ) from wastewater, J. Hazard. Mater. 274(2014) 145-155. |
| [36] | C. Cheng, J.N. Wang, L. Xu, A.M. Li, Preparation of new hyper cross-linked chelating resin for adsorption of Cu2+ and Ni2+ from water, Chin. Chem. Lett. 23(2012) 245-248. |
| [37] | Y.H. Peng, J.N. Wang, X. Yang, C. Cheng, T. Wintgens, Preparation of a novel chelating resin for the removal of Ni2+ from water, Chin. Chem. Lett. 25(2014) 265-268. |
 2016, Vol.27
2016, Vol.27 




