As a new class of inorganic-organic hybrid materials, metalorganic frameworks (MOFs) have attracted great interests not only for their potential applications in gas storage and separation [1, 2, 3, 4], sensor [5, 6, 7], electrochemistry [8, 9] and catalysis [10, 11, 12] but also for fascinating structures as well as abundant topologies [13, 14]. MOFs are crystalline solids with extended network structures, which are comprised of metal-containing nodes and organic ligand linkages linked via coordination bonds [15]. Metalcontaining nodes (known as secondary building units, SBU) play important roles in the design and synthesis of MOFs with various structures and topologies [16, 17, 18]. As a result, many efforts have been devoted to designing and constructing particular SBUs for the synthesis of MOFs with desired structures. For examples, a series of reticular MOFs with homogeneous periodic pores have been obtained based on Cu2(H2O)2(COO)4 SBU [19, 20], and highly stable Zr-based MOFs with various, unique properties have been reported based on Zr6O4(OH)4 SBU [21, 22].
In addition to SBUs, the exploration on organic linkers is also very critical in the design and synthesis of MOFs. Among the diverse organic linkers, carboxylate ligands have been widely used in the construction ofMOFs with specific ormultifunctional properties due to their diverse configurations and coordination modes [23]. As we know, most reported MOFs are based on highly symmetric ligands. However, only rare efforts have been focused on the ligands with lowered symmetry. In thiswork, we designed and synthesized a new desymmetric 4-connected ligand, 5-(2, 6-bis(4-carboxyphenyl)pyridin- 4-yl)isophthalic acid (H4BCPIA). The assembly of BCPIA4- with predesigned 4-connected paddle-wheel plane quadrilateral Cu2(H2O)2(COO)4 or tetrahedral In(COO)4 nodes yielded two threedimensional (3D) MOFs, [Cu2(H2O)2(BCPIA)] (BUT-20, where BUT = Beijing University of Technology) and (Me2NH2)[In(BCPIA)] (BUT-21), respectively. Their structures were determined by singlecrystal X-ray diffractions. In BUT-20, each twelve Cu2(H2O)2(COO)4 SBUs are connected together by six BCPIA4- to form an octahedral cage. Each face of the cage unit is shared by other octahedral cages to form a 3D framework with an Nbo topology. In BUT-21, In(COO)4 nodes are linked by the ligands to form again a 3D framework with two types of open channels along the a axis, and a Unc topology. Obviously, the formation of different 4-connected metal-containing nodes plays a key role in the topological network diversity of the resulting MOFs.
2. Experimental 2.1. Materials and methodsAll general reagents and solvents (AR grade) were commercially available and used as received without further purification. The 1H NMR data were collected on a Mercury 400 spectrometer. Fourier transform infrared (FT-IR) spectra were recorded on an IRAffinity-1 instrument. The powder X-ray diffraction patterns (PXRD) were recorded on a BRUKER D8-Focus Bragg-Brentano X-ray Powder Diffractometer equipped with a Cu sealed tube (λ = 1.54178) at room temperature (r.t.). Simulation of the PXRD spectrum was carried out by the single-crystal data and diffraction-crystal module of theMercury programavailable free of charge via internet at http://www.ccdc.cam.ac.uk/mercury/. Thermogravimetric analysis (TGA) data were obtained on a TGA-50 (SHIMADZU) thermogravimetric analyzer with a heating rate of 10 ℃min-1 under N2 atmosphere. Elemental microanalyses (EA) were performed by VarioMacro cube elementar.
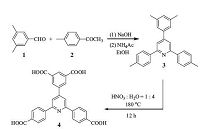
2.2. Synthesis and general characterizations 2.2.1. 5-(2, 6-Bis(4-carboxyphenyl)pyridin-4-yl)isophthalic acid (H4BCPIA)H4BCPIA was synthesized following a procedure as shown in Scheme 1.
|
Download:
|
| Scheme 1.Synthesis procedure for H4BCPIA. | |
4-(3, 5-Dimethylphenyl)-2, 6-di-p-tolylpyridine (3): 3, 5-Dimethyl benzaldehyde (2.0 g, 15mmol) and 4-methyl acetophenone (4.6 g, 34mmol) andNaOH(9.6 g, 240 mmol)were crushedtogether with amortar and pestle for 2 h manually tomake the two reactants reactwell. Then, the obtained yellowpowderwas added to a stirred solution of ammonium acetate (15 g, excess) in ethanol (300 mL). The resulted mixture was heated at reflux for 48 h. Upon cooling to r.t., the precipitatewas filtered, washedwithwater three times, and dried to afford the crude product. It was further purified by flash column chromatography on silica. Elution with n-hexane/ethyl acetate (1:1) gave a white solid of 4-(3, 5-dimethylphenyl)-2, 6-di-ptolylpyridine (yield: 3.4 g, 63.0%.). 1H NMR (CDCl3, 400 MHz): δ 8.14 (d, 4H), 7.85 (s, 2H), 7.35 (t, 6H), 7.14 (s, 1H), and 2.46 (s, 12H).
5-(2, 6-Bis(4-carboxyphenyl)pyridin-4-yl)isophthalic acid (4, H4BCPIA): 0.5 g of 4-(3, 5-dimethylphenyl)-2, 6-di-p-tolylpyridine, 2 mL concentrated HNO3, and 15 mL H2O were added to a 20 mL Teflon-lined stainless steel autoclaves and heated at 180 ℃ for 24 h. After reaction, the product was filtered andwashedwithwater three times. The obtained orange solid of 5-(2, 6-bis(4-carboxyphenyl)pyridin- 4-yl)isophthalic acid (H4BCPIA) was dried at 80 ℃ for 12h (yield: 0.55 g, 83.3%.). 1H NMR (400MHz, DMSO-d6): δ 8.69 (s, 2H), 8.59 (s, 1H), 8.50 (d, 4H), 8.41 (s, 2H), and 8.11 (d, 4H). FT-IR (KBr pellets): 3014 (m), 1695 (s), 1596 (s), 1280 (m), 1238 (m), 858 (w), 759 (w), and 742 (w) (Fig. S3 in Supporting information).
2.2.2. Synthesis of BUT-20 and BUT-21[Cu2(H2O)2(BCPIA)]·nS (BUT-20, S represents non-assignable solvent molecules): A solution of Cu(NO3)2·2.5H2O (20 mg, 0.120 mmol) and H4BCPIA (12 mg, 0.025 mmol) in 3 mL of N, Ndimethylformamide (DMF) was sealed in a 5 mL glass vial and heated at 80 ℃ for 24 h, after cooling down spontaneously, green block crystals were collected (yield: 10 mg). Elemental analysis (EA) for BUT-20, C27H13Cu2NO10 (638): Calcd. C 50.78, H 2.04, N 2.19. Found. C 49.82, H 2.23, N 2.08. FT-IR (KBr pellets): 3367 (m), 1608 (s), 1552 (s), 1402 (s), 862 (w), 788 (w), and 777 (w) (Fig. S3).
(Me2NH2)[In(BCPIA)]·nS (BUT-21): A solution of In(NO3)3·5H2O (10 mg, 0.034 mmol), H4BCPIA (8 mg, 0.017 mmol), and 4 drops of HBF4 (48% water solution) in 1.5 mL of N, N-dimethylacetamide (DMA) was sealed in a 5 mL glass vial and heated at 100 ℃ for 5 days, after cooling down spontaneously, colorless block crystals were obtained (yield: 7 mg). Elemental analysis (EA) for BUT-21, C29H19InN2O8 (637.82): Calcd. C 54.56, H 2.98, N 4.39. Found. C 53.63, H 2.43, N 4.16. FT-IR (KBr pellets): 3430 (m), 1618 (s), 1384 (s), 1083 (s), 863 (w), 790 (w), and 779 (w) (Fig. S3).
2.3. Single-crystal X-ray diffractionSingle-crystal X-ray diffraction data of BUT-20 and -21 were collected on a Bruker-AXS APEX-Ⅱ CCD X-ray diffractometer equipped with a fine-focus sealed-tube X-ray source (graphite monochromaticMo-Karadiation, λ = 0.71073Å , ω-scanswitha 0.5°step). Raw data collection and reduction were performed using APEX2 software [24]. Absorption correctionswere applied using the SADABS routine [25]. The structures were solved by direct methods and refined by full-matrix least-squares on F2 with anisotropic displacement using the SHELXTL software package [26]. All non-H atoms were refined anisotropically during the final cycles. The hydrogen atoms of ligands were calculated at idealized positions with a riding model and refined isotropically. In addition, the hydrogen atomsof coordinatedwatermolecules in BUT-20 were not considered in its structural refinement. There are large solvent accessible pore volumes in the crystals of BUT-20 and -21, which are occupied by highly disordered solvent molecules, or counter-ions (in BUT-21). No satisfactory disorder model for these guest molecules could be achieved, and therefore the SQUEEZE program implemented in PLATON was used to remove the electron densities of these disordered species [27]. Thus, all of the electron densities from free guest molecules have been “squeezed” out. The details of structural refinement can be found in Table S1 in Supporting information, while the selected bond lengths and angles are summarized in Tables S2 and S3 in Supporting information.
3. Results and discussionThe new ligand, H4BCPIA, was synthesized by following a previously reported procedure with modifications (Scheme 1) [28]. The point group of the ligand, BCPIA4- in the structure of BUT-20 and -21 is C2v. Solvothermal reactions of H4BCPIA with Cu(NO3)2·2.5H2O, or In(NO3)3·5H2O in DMF or DMA yielded crystals of BUT-20 and -21, respectively. Their phase purity was checked by PXRD. As shown in Fig. S1 in Supporting information, the experimental PXRD patterns match well with ones simulated from the single-crystal data, indicating the pure phase of them. The TGA plots of the prepared samples of the two MOFs are shown in Fig. S2 in Supporting information, confirming that BUT-20 and -21 are stable up to ca. 260 and 520 ℃, respectively. In addition, as shown in Fig. S3, slight blue-shifts of the characteristic bands of the carbonyl group in the two MOFs compared with that of H4BCPIA could be observed, illustrating the metal coordination of carboxylate groups in the ligand.
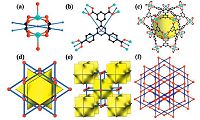
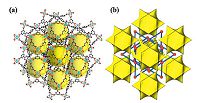
Single-crystal X-ray diffraction analysis reveals that BUT-20 crystallizes in a non-centrosymmetric hexagonal space group R- 3m and possesses an extended 3D framework structure. In BUT-20, both CuⅡ atoms lie on a twofold axis and each is coordinated by one water molecule and four oxygen atoms from carboxyl groups of four different BCPIA4- ligands. Two CuⅡ atoms are linked by four carboxylate groups from different ligands into a classical Cu2(H2O)2(COO)4 paddle-wheel SBU with a Cu-Cu distance of 2.6599(5) Å (Fig. 1a). As shown in Fig. 1b, four carboxylate groups in the BCPIA4- ligand only exhibit a bis-monodentate coordination mode. It should be pointed out that the prominent structural feature of BUT-20 is the presence of an interesting, cavity-like substructures, where twelve dimeric Cu2(COO)4 SBUs are connected together by six BCPIA4- ligands to form an octahedral cage, which can be filled by a ball with the diameter of about 7.1Å (Fig. 1c and d). Moreover, each octahedral cage is surrounded by eight equal ones (Fig. 1e). Each face of the octahedral cage is shared by other octahedral cages to extend to a 3D framework (Fig. 2). After removal of free solvent molecules, the total solventaccessible volume of BUT-20 is estimated to be 73.1% by PLATON [27]. From the topological viewpoint, each Cu2(H2O)2(COO)4 SBU and BCPIA4- ligand can be viewed as 4-connected nodes (Fig. 1a and b), the overall 3D structure of BUT-20 can thus be simplified as a 4-connected net with the point symbol of (64·82), corresponding to Nbo-type topology (Fig. 1f). The reported Nbo-type MOFs in the literature such as NOTT-105 [29], PCN-14 [30], MOF-505 [31], UTSA-76 [32] and UTSA-80 [33] are well-known and they all crystallize in space group R-3m. In our case, BUT-20 crystallizes in a lower space group R-3m with the loss of an inversion center.
|
Download:
|
| Fig. 1.(a) Viewof the [Cu2(H2O)2(CO2)4] paddle-wheel SBU; (b) coordinationmode of BCPIA4- ligand; (c) the cage-type substructure; (d) octahedron cavity in BUT-20; (e) natural tiling of BUT-20 showing the packing of the octahedron cavities in BUT-20; and (f) topological representation of BUT-20 (H atoms on the ligands are omitted for clarity). | |
|
Download:
|
| Fig. 2.(a) The 3D framework structure of BUT-20 with highlighted cages and (b) the natural tiling of BUT-20 viewed along the [111] direction (H atoms on the ligands are omitted for clarity). | |
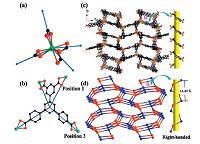
BUT-21 crystallizes in an orthorhombic chiral space group of P212121, which suggests the presence of 21 helical chains along the a, b and c axes. In the structure of BUT-21, the InⅢ ion adopts a tetrahedral linkage geometry through coordinating to eight oxygen atomsfromfour carboxylate groups of four different BCPIA4- ligands (Fig. 3a). The In-O distances range from 2.2617(1)Å to 2.2905(1)Å , which are comparable to those of reported indium complexes [34]. As shown in Fig. 3b, the carboxylate groups in BCPIA4- ligand exhibit a chelating coordination mode, where each one coordinates to one InⅢ ion. Furthermore, in the structure there exist two different open channels along the a axis with rectangular windows having dimensions of 8.9 × 17.5Å and 11.8 × 18.6Å (atomto atom distance), respectively (Fig. 3c) and along the a axis, there exists a right-handed helical chain (Fig. 3c and d). Two carboxylate groups in position 1 and position 2 of the BCPIA4- ligand (illustrated in Fig. 3b) connect two adjacent In(COO)4 entities to form 21 righthanded helical chains along a axis (the helical pitch is 14.49Å , given by one full rotation around the 21 helical axis). Furthermore, each large channel is surrounded by four small channels, while the small channel is surrounded by four large channels to extend to a 3D framework (Fig. 3c). After removal of free solvent molecules, the total solvent-accessible volume of BUT-21 is estimated to be 75.8% by PLATON [27]. From the topological viewpoint, each In(COO)4 node and BCPIA4- ligand can be viewed as 4-connected nodes, and the 3D structure of BUT-21 can thus be simplified as a 4-connected net with the point symbol of (66), corresponding to a Unc-type topology (Fig. 3d). It should also be pointed out that in the pore of BUT-21, there should exist counter-cations balancing the charge of the framework. However, these counter-cations are highly disordered, and could not be identified by single-crystal X-ray diffraction analysis. EA analysis was then carried out, which confirmed extra N content inside the pore. The result implies the presence of (CH3)2NH2+, which is a common by-product in solvothermal reactions with DMF or DMA as solvent [35]. Furthermore, the TGA curve showed a 7.1% weight loss between 200 ℃ and 340 ℃, which corresponds well to the presence of one (CH3)2NH2+ ion per formula.
|
Download:
|
| Fig. 3.(a) View of the [In(CO2)4] node; (b) coordination mode of the BCPIA4- ligand; (c) 3D framework structure; and (d) topological representation of BUT-21 (H atoms on the ligands are omitted for clarity). | |
As described above, the reaction between BCPIA4- ligand and CuⅡ or InⅢ under solvothermal conditions gives two MOFs, each with distinct network structures. The generation of these different networks could be mainly attributed to the distinct metal-containingnode represented in the structures. In BUT-20, the BCPIA4- ligand connects with the plane quadrilateral Cu2(H2O)2(COO)4 SBU, while in BUT-21, the BCPIA4- ligand connects with the tetrahedral In(COO)4 node.
4. ConclusionIn summary, we have designed and synthesized a new desymmetric 4-connected ligand, H4BCPIA, and was used to construct novel MOFs with two predesigned 4-connected metalcontaining nodes, plane quadrilateral Cu2(H2O)2(COO)4 and tetrahedral In(COO)4. As a result, two new MOFs with fascinating structures of Nbo and Unc topologies were created. Clearly, the linkage configurations of the metal-containing nodes play important roles in determining the framework structures of the resulting MOFs. Currently, investigations involving desymmetric ligands in the design and construction of new MOFs are in progress in our laboratory.
AcknowledgmentThisworkwasfinanciallysupportedbytheNSFC(Nos.21322601, 21271015, 21406006, andU1407119) and ProgramforNewCentury Excellent Talents in University (No. NCET-13-0647).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at: http://dx.doi.org/10.1016/j.cclet.2015.12.034.
| [1] | J.T. Jia, L. Wang, F.X. Sun, et al., The adsorption and simulated separation of light hydrocarbons in isoreticular metal-organic frameworks based on dendritic ligands with different aliphatic side chains, Chemistry 20(2014) 9073-9080. |
| [2] | L. Qin, Z.M. Ju, Z.J. Wang, et al., Interpenetrated metal-organic frameworks with selective gas adsorption and luminescent properties, Cryst. Growth Des. 14(2014) 2742-2746. |
| [3] | J.R. Li, J. Sculley, H.C. Zhou, Metal-organic frameworks for separations, Chem. Rev. 112(2012) 869-932. |
| [4] | D. Liu, J.P. Lang, B.F. Abrahams, Highly efficient separation of a solid mixture of naphthalene and anthracene by a reusable porous metal-organic framework through a single-crystal-to-single-crystal transformation, J. Am. Chem. Soc. 133(2011) 11042-11045. |
| [5] | S. Pramanik, C. Zheng, X. Zhang, T.J. Emge, J. Li, New microporous metal-organic framework demonstrating unique selectivity for detection of high explosives and aromatic compounds, J. Am. Chem. Soc. 133(2011) 4153-4155. |
| [6] | S.R. Zhang, D.Y. Du, J.S. Qin, et al., A fluorescent sensor for highly selective detection of nitroaromatic explosives based on a 2D, extremely stable, metalorganic framework, Chemistry 20(2014) 3589-3594. |
| [7] | M.M. Chen, X. Zhou, H.X. Li, X.X. Yang, J.P. Lang, Luminescent two-dimensional coordination polymer for selective and recyclable sensing of nitroaromatic compounds with high sensitivity in water, Cryst. Growth Des. 15(2015) 2753-2760. |
| [8] | L. Kang, S.X. Sun, L.B. Kong, J.W. Lang, Y.C. Luo, Investigating metal-organic framework as a new pseudo-capacitive material for supercapacitors, Chin. Chem. Lett. 25(2014) 957-961. |
| [9] | X.Y. Dong, X.P. Hu, H.C. Yao, et al., Alkaline earth metal (Mg, Sr, Ba)-organic frameworks based on 2,20,6,60-tetracarboxybiphenyl for proton conduction, Inorg. Chem. 53(2014) 12050-12057. |
| [10] | D.Y. Shi, C. He, B. Qi, et al., Merging of the photocatalysis and copper catalysis in metal-organic frameworks for oxidative C-C bond formation, Chem. Sci. 6(2015) 1035-1042. |
| [11] | K. Mo, Y.H. Yang, Y. Cui, A homochiral metal-organic framework as an effective asymmetric catalyst for cyanohydrin synthesis, J. Am. Chem. Soc. 136(2014) 1746-1749. |
| [12] | D. Liu, Z.G. Ren, H.X. Li, et al., Single-crystal-to-single-crystal transformations of two three-dimensional coordination polymers through regioselective[2+2] photodimerization reactions, Angew. Chem. Int. Ed. 49(2010) 4767-4770. |
| [13] | K.H. He, Y.W. Li, Y.Q. Chen, Z. Chang, A new 8-connected self-penetrating metalorganic framework based on dinuclear cadmium clusters as secondary building units, Chin. Chem. Lett. 24(2013) 691-694. |
| [14] | F. Wang, H.R. Fu, J. Zhang, Homochiral metal-organic framework with intrinsic chiral topology and helical channels, Cryst. Growth Des. 15(2015) 1568-1571. |
| [15] | Y. Han, J.R. Li, Y.B. Xie, G.S. Guo, Substitution reactions in metal-organic frameworks and metal-organic polyhedra, Chem. Soc. Rev. 43(2014) 5952-5981. |
| [16] | E. Lee, Y. Kim, J. Heo, K.M. Park, 3D metal-organic framework based on a lower-rim aci δ-functionalized calix[4] arene:crystal-to-crystal transformation upon lattice solvent removal, Cryst. Growth Des. 15(2015) 3556-3560. |
| [17] | Q.G. Zhai, C.Z. Lu, X.Y. Wu, S.R. Batten, Coligand modulated six-, eight-, and tenconnected Zn/Cd-1, 2, 4-triazolate frameworks based on mono-, bi-, tri-, penta-, and heptanuclear cluster units, Cryst Growth Des. 7(2007) 2332-2342. |
| [18] | T.P. Hu, W.H. Bi, X.Q. Hu, X.L. Zhao, D.F. Sun, Construction of metal-organic frameworks with novel {Zn8O13} SBU or chiral channels through in situ ligand reaction, Cryst. Growth Des. 10(2010) 3324-3326. |
| [19] | D.Q. Yuan, D. Zhao, D.F. Sun, H.C. Zhou, An isoreticular series of metal-organic frameworks with dendritic hexacarboxylate ligands and exceptionally high gasuptake capacity, Angew. Chem. Int. Ed. 49(2010) 5357-5361. |
| [20] | D. Zhao, D.Q. Yuan, D.F. Sun, H.C. Zhou, Stabilization of metal-organic frameworks with high surface areas by the incorporation of mesocavities with microwindows, J. Am. Chem. Soc. 131(2009) 9186-9188. |
| [21] | B. Wang, H.L. Huang, X.L. Lv, et al., Tuning CO2 selective adsorption over N2 and CH4 in UiO-67 analogues through ligand functionalization, Inorg. Chem. 53(2014) 9254-9259. |
| [22] | K.K. Wang, H.Q. Huang, W.J. Xue, et al., An ultrastable Zr metal-organic framework with a thiophene-type ligand containing methyl groups, CrystEngComm 17(2015) 3586-3590. |
| [23] | Y. Yan, S.H. Yang, A.J. Blake, M. Schrö der, Studies on metal-organic frameworks of Cu(Ⅱ) with isophthalate linkers for hydrogen storage, Acc. Chem. Res. 47(2014) 296-307. |
| [24] | Bruker AXS Inc., APEX Software Package, Bruker Molecular Analysis Research Tool Version 2008.4, Bruker AXS Inc., Madison, WI, 2008. |
| [25] | G.M. Sheldrick, SADABS Program for Absorption Correction of Area Detector Frames, Bruker AXS, Inc., Madison, WI, 2001. |
| [26] | G.M. Sheldrick, SHELXTL-97 Structure Determination Software Suite, Bruker AXS, Inc., Madison, WI, 2008. |
| [27] | A.L. Spek, Single-crystal structure validation with the program PLATON, J. Appl. Crystallogr. 36(2003) 7-13. |
| [28] | J.X. Yang, X.T. Tao, C.X. Yuan, et al., A facile synthesis and properties of multicarbazole molecules containing multiple vinylene bridges, J. Am. Chem. Soc. 127(2005) 3278-3279. |
| [29] | X. Lin, I. Telepeni, A.J. Blake, et al., High capacity hydrogen adsorption in Cu(Ⅱ) tetracarboxylate framework materials:the role of pore size, ligand functionalization, and exposed metal sites, J. Am. Chem. Soc. 131(2009) 2159-2171. |
| [30] | S.Q. Ma, D.F. Sun, J.M. Simmons, et al., Metal-organic framework from an anthracene derivative containing nanoscopic cages exhibiting high methane uptake, J. Am. Chem. Soc. 130(2008) 1012-1016. |
| [31] | B.L. Chen, N.W. Ockwig, A.R. Millward, D.S. Contreras, O.M. Yaghi, High H2 adsorption in a microporous metal-organic framework with open metal sites, Angew. Chem. Int. Ed. 44(2005) 4745-4749. |
| [32] | B. Li, H.M. Wen, H.L. Wang, et al., A porous metal-organic framework with dynamic pyrimidine groups exhibiting record high methane storage working capacity, J. Am. Chem. Soc. 136(2014) 6207-6210. |
| [33] | H.M. Wen, B. Li, D.Q. Yuan, et al., A porous metal-organic framework with an elongated anthracene derivative exhibiting a high working capacity for the storage of methane, J. Mater. Chem. A 2(2014) 11516-11522. |
| [34] | L.B. Sun, H.Z. Xing, Z.Q. Liang, J.H. Yu, R.R. Xu, A 4+4 strategy for synthesis of zeolitic metal-organic frameworks:an indium-MOF with SOD topology as a lightharvesting antenna, Chem. Commun. 49(2013) 11155-11157. |
| [35] | J.M. Gu, S.J. Kim, Y. Kim, S. Huh, Structural isomerism of an anionic nanoporous In-MOF with interpenetrated diamond-like topology, CrystEngComm 14(2012) 1819-1824. |
 2016, Vol.27
2016, Vol.27 


