b State Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China;
c College of Physics, Jilin University, Changchun 130012, China
Metal-organic frameworks (MOFs) with metal ions as nodes and organic ligands as linkers,have received extensive attention because of their diverse structures and fascinating properties [1, 2, 3, 4, 5], which have widely prospective applications in many fields, such as gas storage and separation, heterogeneous catalysis, drug delivery [6, 7, 8, 9, 10]. Particularly, luminescent MOFs with amazing optical properties have been considered as one of the most promising class of functional materials for sensing application [11, 12]. Indeed, fluorescent probe provides a prospective alternative to handle with analytical issues in environmental and biomedical sciences [13]. The acquirements for an ideal fluorescent probe may include stable, simple detection, high signal output, low cost, and good reliability [14, 15, 16]. The amazing nature of MOFs makes them excellent candidates as sensory materials superior to inorganic and organic semiconductors in respects of sensitivity and selectivity [17, 18]. The advantages of MOFs as sensing platform cover two considerations: (1) their structural and chemical tunability offers good selectivity due to different pore sizes and host-guest interactions; (2) their high internal surface areas with high concentration of analytes contributes to decreasing detection limits and increasing high sensitivity. Taking advantages of the excellent optical properties of luminescent MOFs, considerable efforts have been focused on various MOF materials targeted for recognition of cations, anions, temperature, humidity, organic compounds and solvents [19, 20, 21]. A variety of MOF-based luminescent sensors have been built up for the detection of Cu2+, F-, acetone, DNT and so on, and we are quite impressed by their prominent advantages like easy manipulation and good reproducibility [22, 23].
Rapid and readily detection of organic solvents are of significance with widespread social concerns straightly related to environmental and health problems [24, 25]. On the other hand, iron ion, one of the most important elements in human body, take essential part in a number of vital biological systems [26, 27]. Luminescent MOFs perform sufficient interactions between the host frameworks and the guest molecules, such as hydrogen bonding capability, π-π stacking and electrostatic interaction [28, 29]. Most of past research works on MOF sensors have been concentrated on monofunctional sensing of analytes [30, 31]. However, MOF with multiple functions responsible for plenty of analytes have been called for more and more widely practical demands [32]. Cheng and coworkers have developed two novel isostructural lanthanide MOFs, which displayed high-sensitivity detection ability for benzene and acetone, Cu2+, and CrO42- [33]. Su et al. have constructed a anionic Zn-containing MOF for luminescence sensing of Ln3+ ions and detection of nitrobenzene [34]. Liu group employed two kinds of Cd-Ln heterometallic MOFs as sensitive sensors not only for solvents but also for Mn2+ [35]. In this work, a semi-rigid ligand 4, 4′, 4″-(methylsilanetriyl)tribenzoic acid (H3L) was selected as a building block to construct structures. Solvothermal reaction of H3L and Cd2+ ions in a mixed solvent system (DMF/H2O) at 100 ℃ afforded a novel compound CdL{[Cd3(L)2(H2O)6]·1.5DMF}. The photoluminescent properties of CdL were carefully studied in detail. Furthermore, CdL was employed as a luminescent sensor in the detection of organic solvents and metal ions. It is quite impressive that CdL displayed excellent sensing performance as a multi-responsive luminescent sensor for acetone and Fe3+ ion.
2. Experimental 2.1. Materials and characterizationAll reagents were purchased commercially and used without further purification. Elemental analyses of C, H, N, and Cd ions in the solid samples were tested on a VarioEL analyzer (Elementar Analysensysteme GmbH) and via inductively coupled plasma (ICP) atomic emission spectrometric analysis (POEMS, TJA), respectively. Thermogravimetric analysis (TGA) was performed on a SDT 2960 simultaneous DSC-TGA of TA instruments up to 800 ℃, and the heating rate was 10 ℃ min-1 under an air flow of 100 mL min-1. Powder X-ray power diffraction (XRD) patterns were carried out on a D8 Focus (Bruker) diffractometer with Cu Kα radiation Field-emission (λ = 0.15405 nm, continuous, 40 kV, 40 mA, increment = 0.02°).
2.2. Synthesis of CdLH3L (0.1 mmol, 40.65 mg), Cd(NO3)3·4H2O (0.1mmol, 30.85 mg), DMF (4 mL), and H2O (4 mL) were placed in a 20 mL sealed glass vial, which was kept at 80 ℃ for three days. After cooling to room-temperature, the precipitates were washed by DMF and water for three times, and finally the colorless block crystals were obtained, yield 27.8 mg (67.1%, based on Cd2+ ion).
2.3. X-ray crystal structure determinationSuitable crystals with dimension of 0.21×0.20×0.24 mm3 for CdL, was selected for single crystal X-ray diffraction analysis. Crystallographic data were collected at 296 K on a Bruker Apex Ⅱ CCD diffractometer with graphite monochromated Mo-Ka radiation (λ = 0.71073Å). Data processing was accomplished with the SAINT program. The structure was solved by direct methods and refined on F2 by full-matrix least squares using SHELXTL-97. Nonhydrogen atoms were refined with anisotropic displacement parameters during the final cycles. All hydrogen atoms of the organic molecule were placed by geometrical considerations and were added to the structure factor calculation. Isolated solvents within the channels were not crystallographically well-defined and could not be modeled properly, the DMF molecule was removed by the SQUEEZE routine in PLATON, which was determined by elemental analysis and thermogravimetric analysis. The summary of the crystallographic data for CdL is listed in Tables S1 and S2 in Supporting information.
2.4. Fluorescent measurementsThe luminescence spectra were recorded at room temperature on a Hitachi F-7000 fluorescence spectrophotometer with a 150W Xenon lamp as the excitation source at room temperature. The photomultiplier tube (PMT) voltage was 700 V, the scan speed was 1200 nm min-1, the slit width of excitation and emission is 5 nm. Activated CdL was prepared by thermal activation of the corresponding compound at 120 ℃ in vacuum for 4 h. For cation sensing, activated CdL powders (5 mg each) were introduced into aqueous solutions of metal nitrates (Mn+ = K+, Ag+, Zn2+, Ni2+, Mn2+, Co2+, Cu2+, Hg2+, Pb2+, Ca2+, Mg2+, Ba2+, Sr2+, Fe2+, Fe3+, Al3+) and then immersed for two days before fluorescence studies. For solvents sensing, activated CdL powders (5 mg each) were dispersed in various solvents (3 mL each), including DMF, methanol, ethanol, acetone, 1-propanol, 2-propanol, dichloromethane, cloroform, acetonitrile, n-hexane, and H2O. The emulsions were then aged for two days before further luminescence tests.
3. Results and discussionThe experimental and simulated X-ray powder diffraction patterns of the bulk materials CdL are shown in Fig. S1 in Supporting information. They match perfectly to each other, confirming the phase purity of the as-synthesized products. Furthermore, the TGA result reveals that CdL shows a weight loss of 8.21 wt% in the range of 90 ℃ and 160 ℃, corresponding to the loss of the DMF molecule in the channels (calcd. 8.33 wt%). No further weight loss was observed until 340 ℃, at which the compound starts to decompose quickly (Fig. S2 in Supporting information). Thus CdL was formulated as [Cd3(L)2(H2O)6]·1.5DMF based on the TGA and single-crystal X-ray diffraction study. ICP analysis gives the content of Cd as 25.46 wt%, and elemental analyses give the contents of C, H and N as 44.20, 1.08 and 1.07 wt%, which are in agreement with the calculated values (Cd, 25.37 wt%; C, 44.28 wt%; H, 1.00 wt%; N, 1.05 wt%) based on the formula given by single-crystal X-ray diffraction and TGA analyses.
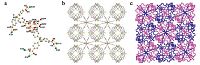
The structural information of CdL has been studied by singlecrystal X-ray diffraction measurement, which reveals CdL crystallizes in the cubic space group Pn-3. The asymmetric unit of CdL consists of two crystallographocally independent Cd sites and two distinct ligands. As depicted in Fig. 1a, Cd1 atom has a distorted dodecahedral coordination environment surrounded by eight oxygen atoms from four chelating carboxylate groups in four ligands, whereas Cd2 atom is six coordinated with a distorted octahedral geometry defined by two oxygen atoms from two different ligands and four coordinated water molecules. The Cd1 connects to neighboring Cd2 via edge-sharing to form a dinuclear cluster. As shown in Fig. 1b, such dimetallic building units are further linked together with carboxylic ligands resulting in a threedimensional framework with 2-fold interpenetration. A topological analysis of the framework has been performed by TOPOS in order to have a deeply understanding of the structure of CdL. It indicates that the framework is a 3, 4-connected pto net with point symbol of {83 }4{86}3, where the metallic SBU is considered as an four-connected node, and ligand is considered as a threeconnected node during simplification (Fig. 1c) [36].
|
Download:
|
| Fig. 1.(a) Ortep representation of CdL (cadmium, green; carbon, gray; silicon, yellow; oxygen, red). Thermal ellipsoids were drawn at 30% probability. (b) The independent single net in the interpenetrating framework of CdL along the [0 0 1] directions. (c) Representation of 2-fold interpenetrating net of CdL along the [0 0 1] directions. Hydrogen atoms were omitted for clarity. | |
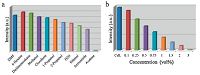
The emission and excitation spectra of CdL were measured in the solid state. As shown in Fig. S3 in Supporting information, when excitated at 312 nm, the fluorescence spectra of CdL, exhibits one emission maximum at 343 nm corresponding to the emission of ligand (Fig. S4 in Supporting information). Generally, Fe3+ ion, which is one of the most significant elements in human body, take an essential part in human body and biological system. Analytical technique based on fluorescence phenomena shows great advantages towards cation sensing due to its simplicity, sensitivity and low cost. In this work, the luminescence behavior of CdL towards cations was investigated to evaluate its potential as a luminescent sensor. Activated samples of CdL were introduced into an aqueous solutions (0.01 mol L-1) containing various cations (K+, Ag+, Zn2+, Ni2+, Mn2+, Co2+, Cu2+, Hg2+, Pb2+, Ca2+, Mg2+, Ba2+, Sr2+, Fe2+, Fe3+, Al3+). The luminescent properties are recorded and shown in Fig. 2. It is can been seen from the spectra that most metal ions, such as K+ and Ca2+, have subtle influence on the luminescence intensities of CdL, while a few metal ions, like Ag+ and Cu2+, suppress the luminescence intensity partly. Particularly, the luminescence of the compound is completely quenched in the presence of Fe3+ ion. Further experiments indicate that the introducing trace of Fe3+ could remarkably decrease the luminescence, and the luminescence of CdL is totally quenched when the concentration of Fe3+ ion increases to 10 mmol L-1. Moreover, XRD measurements reveal that the MOF basic framework remains after treatment with various cations (Fig. 3). It is deduced that the quenching effect of CdL towards Fe3+ ion is mainly attributed to the physical interactions between Fe3+ ion and carboxylic acid, which is generally confirmed by other studies [11, 37]. The outstanding performance of CdL highlights that it can be considered as a highly selective and exclusive fluorescent probe via the fluorescence quenching phenomenon.

|
Download:
|
| Fig. 2.(a) A comparison of the luminescence intensity of CdL at 343 nm for different metal ions, monitored at 312 nm. (b) A comparison of the luminescence intensity of CdL at 343 nm with different concentrations of Fe3+ ion, monitored at 312 nm. | |

|
Download:
|
| Fig. 3.Powder XRD patterns of CdL treated with different cations. | |
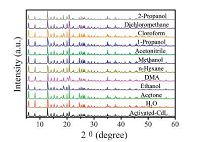
On the other hand, the interesting porous characteristics of CdL encourage us to use them as hosts for sensitizing and encapsulating common solvents, which is of high importance for widely environmental and health concerns. To examine the potential application of CdL for the solvents, CdL powders were immersed in various solvents, like DMF, methanol, ethanol, acetone, 1-propanol, 2-propanol, dichloromethane, cloroform, acetonitrile, n-hexane, and H2O, treated with ultrasonication, and then aged for two days to form a stable emulsion before fluorescence measurements were recorded. The photoluminescence spectra of CdL in different solvents were tested and shown in Fig. 4a. The most intriguing feature is that the photoluminescence spectra are largely dependent on the types of organic solvents. When excited at 312 nm, emissions are observed in most organic solvents except acetone, which exhibits the most significant quenching effect towards MOF luminescence. Taking CdL dispersed in 1-propanol as the standard emulsion, the acetone content is gradually increased to test investigate the emission response of the compound (Fig. 4b). It is obvious that the luminescence intensities of CdL emulsions continuously decrease with the introduction of acetone and the luminescence of the emulsion was completely undetectable by instrument when the acetone content reaches 3 vol%. The decreasing tendency of the fluorescence intensity of CdL at 343 nm versus the acetone content are in good agreement with a first-order exponential decay (Fig S5 in Supporting information), revealing a diffusion-controlled process of the acetone quenching phenomenon. Powder XRD patterns were measured to study the structural information of CdL before and after the solvents treating, which indicates that the structure of the material is maintained (Fig. 5). The competition of the absorbed energy between the solvents and organic ligand results in a decrease in the luminescence intensity [15, 20].
|
Download:
|
| Fig. 4.(a) A comparison of the luminescence intensity of CdL at 343 nm in different solvents, monitored at 312 nm. (b) A comparison of the luminescence intensity of CdL at 343 nm with different concentrations of acetone, monitored at 312 nm. | |
|
Download:
|
| Fig. 5.Powder XRD patterns of CdL treated with different solvents. | |
In conclusion,a Cd-based metal-organic framework has been synthesized under solvothermal condition adopting a semi-rigid carboxylic acid. The crystal structure of the compound has been carefully studied,which clearly reveals a 2-fold interpenetrating three-dimensional framework. The photoluminescence of CdL enables its potential utility as luminescent sensor. Systematical experiments has certainly demonstrated that the perfect performance of CdL in the detection of acetone and Fe3+ ion,which implies CdL could be regarded as a successfulmulti-responsive luminescent sensor for detection of organic solvents and metal ions.
AcknowledgmentsThis work was supported by National Natural Science Foundation of China (Nos. 21171162,21471144), Jilin Province Youth Foundation (No. 20130522132JH), Jilin Province Natural Science Foundation (No. 20150101181JC), Changchun Science and Technology Plan (No. 2013059).
| [1] | N.W. Ockwig, O. Delgado-Friedrichs, M. O'Keeffe, O.M. Yaghi, Reticular chemistry:occurrence and taxonomy of nets and grammar for the design of frameworks, Acc. Chem. Res. 38(2005) 176-182. |
| [2] | J. Lee, O.K. Farha, J. Roberts, et al., Metal-organic framework materials as catalysts, Chem. Soc. Rev. 38(2009) 1450-1459. |
| [3] | W. Zhang, Y.L. Hu, J. Ge, H.L. Jiang, S.H. Yu, A facile and general coating approach to moisture/water-resistant metal-organic frameworks with intact porosity, J. Am. Chem. Soc. 136(2014) 16978-16981. |
| [4] | L.Q. Ma, J.M. Falkowski, C. Abney, W.B. Lin, A series of isoreticular chiral metal-organic frameworks as a tunable platform for asymmetric catalysis, Nat. Chem. 2(2010) 838-846. |
| [5] | C.A. Bauer, T.V. Timofeeva, T.B. Settersten, et al., Influence of connectivity and porosity on ligand-based luminescence in zinc metal-organic frameworks, J. Am. Chem. Soc. 129(2007) 7136-7144. |
| [6] | J.R. Li, R.J. Kuppler, H.C. Zhou, Selective gas adsorption and separation in metal-organic frameworks, Chem. Soc. Rev. 38(2009) 1477-1504. |
| [7] | P. Horcajada, C. Serre, M. Vallet-Regí, et al., Metal-organic frameworks as efficient materials for drug delivery, Angew. Chem. Int. Ed. 45(2006) 5974-5978. |
| [8] | A. Corma, From microporous to mesoporous molecular sieve materials and their use in catalysis, Chem. Rev. 97(1997) 2373-2420. |
| [9] | H.L. Jiang, Y. Tatsu, Z.H. Lu, Q. Xu, Non-, micro-, and mesoporous metal-organic framework isomers:reversible transformation, fluorescence sensing, and large molecule separation, J. Am. Chem. Soc. 132(2010) 5586-5587. |
| [10] | W. Wang, Y. Yuan, F.X. Sun, G.S. Zhu, Targeted synthesis of novel porous aromatic frameworks with selective separation of CO2/CH4 and CO2/N2, Chin. Chem. Lett. 25(2014) 1407-1410. |
| [11] | Y.J. Cui, Y.F. Yue, G.D. Qian, B.L. Chen, Luminescent functional metal-organic frameworks, Chem. Rev. 112(2012) 1126-1162. |
| [12] | L.E. Kreno, K. Leong, O.K. Farha, et al., Metal-organic framework materials as chemical sensors, Chem. Rev. 112(2012) 1105-1125. |
| [13] | Z.G. Xie, L.Q. Ma, K.E. deKrafft, A. Jin, W.B. Lin, Porous phosphorescent coordination polymers for oxygen sensing, J. Am. Chem. Soc. 132(2010) 922-923. |
| [14] | B. Zhao, H.L. Gao, X.Y. Chen, et al., A Promising MgⅡ-Ion-selective luminescent probe:structures and properties of Dy-Mn polymers with high symmetry, Chem. Eur. J. 12(2006) 149-158. |
| [15] | F.Y. Yi, W.T. Yang, Z.M. Sun, Highly selective acetone fluorescent sensors based on microporous Cd(Ⅱ) metal-organic frameworks, J. Mater. Chem. 22(2012) 23201-23209. |
| [16] | T. Wen, D.X. Zhang, J. Liu, R. Lin, J. Zhang, A multifunctional helical Cu(I) coordination polymer with mechanochromic, sensing and photocatalytic properties, Chem. Commun. 49(2013) 5660-5662. |
| [17] | J. Rocha, L.D. Carlos, F.A. Almeida Paz, D. Ananias, Luminescent multifunctional lanthanides-based metal-organic frameworks, Chem. Soc. Rev. 40(2011) 926-940. |
| [18] | Z.C. Hu, B.J. Deibert, J. Li, Luminescent metal-organic frameworks for chemical sensing and explosive detection, Chem. Soc. Rev. 43(2014) 5815-5840. |
| [19] | Q.K. Liu, J.P. Ma, Y.B. Dong, Reversible adsorption and separation of aromatics on CdⅡ-triazole single crystals, Chem. Eur. J. 15(2009) 10364-10368. |
| [20] | B.L. Chen, Y. Yang, F. Zapata, et al., Luminescent open metal sites within a metal-organic framework for sensing small molecules, Adv. Mater. 19(2007) 1693-1696. |
| [21] | Y. Chen, S.Q. Ma, Microporous lanthanide metal-organic frameworks, Rev. Inorg. Chem. 32(2012) 81-100. |
| [22] | S.N. Zhao, L.J. Li, X.Z. Song, et al., Lanthanide ion codoped emitters for tailoring emission trajectory and temperature sensing, Adv. Funct. Mater. 25(2015) 1463-1469. |
| [23] | Y. Zhou, B. Yan, F. Lei, Postsynthetic lanthanide functionalization of nanosized metal-organic frameworks for highly sensitive ratiometric luminescent thermometry, Chem. Commun. 50(2014) 15235-15238. |
| [24] | C. Zhan, S. Ou, C. Zou, M. Zhao, C.D. Wu, A luminescent mixed-lanthanide-organic framework sensor for decoding different volatile organic molecules, Anal. Chem. 86(2014) 6648-6653. |
| [25] | M. Tu, S. Wannapaiboon, K. Khaletskaya, R.A. Fischer, Engineering zeolitic-imidazolate framework (ZIF) thin film devices for selective detection of volatile organic compounds, Adv. Funct. Mater. 25(2015) 4470-4479. |
| [26] | H. Xu, H.C. Hu, C.S. Cao, B. Zhao, Lanthanide organic framework as a regenerable luminescent probe for Fe3+, Inorg. Chem. 54(2015) 4585-4587. |
| [27] | Z. Chen, Y.W. Sun, L.L. Zhang, et al., A tubular europium-organic framework exhibiting selective sensing of Fe3+ and Al3+ over mixed metal ions, Chem. Commun. 49(2013) 11557-11559. |
| [28] | Y.Q. Lan, H.L. Jiang, S.L. Li, Q. Xu, Solvent-induced controllable synthesis, singlecrystal to single-crystal transformation and encapsulation of Alq3 for modulated luminescence in (4,8)-connected metal-organic frameworks, Inorg. Chem. 51(2012) 7484-7491. |
| [29] | D.X. Ma, B.Y. Li, X.J. Zhou, et al., A dual functional MOF as a luminescent sensor for quantitatively detecting the concentration of nitrobenzene and temperature, Chem. Commun. 49(2013) 8964-8966. |
| [30] | Y. Li, S.S. Zhang, D.T. Song, A luminescent metal-organic framework as a turn-on sensor for DMF vapor, Angew. Chem. Int. Ed. 52(2013) 710-713. |
| [31] | M.M. Chen, X. Zhou, H.X. Li, X.X. Yang, J.P. Lang, Luminescent two-dimensional coordination polymer for selective and recyclable sensing of nitroaromatic compounds with high sensitivity in water, Cryst. Growth Des. 15(2015) 2753-2760. |
| [32] | S. Sanda, S. Parshamoni, S. Biswas, S. Konar, Highly selective detection of palladium and picric acid by a luminescent MOF:a dual functional fluorescent sensor, Chem. Commun. 51(2015) 6576-6579. |
| [33] | J.M. Zhou, W. Shi, H.M. Li, H. Li, P. Cheng, Experimental studies and mechanism analysis of high-sensitivity luminescent sensing of pollutional small molecules and ions in Ln4O4 cluster based microporous metal-organic frameworks, J. Phys. Chem. C 118(2014) 416-426. |
| [34] | J.S. Qin, S.J. Bao, P. Li, et al., A stable porous anionic metal-organic framework for luminescence sensing of Ln3+ ions and detection of nitrobenzene, Chem. Asian J. 9(2014) 749-753. |
| [35] | J.X. Ma, X.F. Huang, X.Q. Song, W.S. Liu, Assembly of framework-isomeric 4d-4f heterometallic metal-organic frameworks with neutral/anionic micropores and guest-tuned luminescence properties, Chem. Eur. J. 19(2013) 3590-3595. |
| [36] | V.A. Blatov, TOPOS, A Multipurpose Crystallochemical Analysis with the Program Package, Samara State University, Russia, 2004. |
| [37] | Y.Q. Xiao, Y.J. Cui, Q. Zheng, et al., A microporous luminescent metal-organic framework for highly selective and sensitive sensing of Cu2+ in aqueous solution, Chem. Commun. 46(2010) 5503-5505. |
 2016, Vol.27
2016, Vol.27 


