b School of Chemistry & Chemical Engineering, Linyi University, Linyi 276005, China
In the past decade, the fabrication of coordination polymers (CPs) and metal-organic frameworks (MOFs) with various nanostructures have attracted considerable interests due to their novel framework structures, topologies and interesting properties [1, 2, 3, 4, 5]. More recently, the use of CPs and MOFs with specific morphology as precursors for the synthesis of nanoscale metal oxides, metal oxide/amorphous carbon with interesting properties has received great attention [6, 7, 8, 9, 10].
Indium oxide (In2O3), which is an n-type semiconductor with a direct band gap of 3.55-3.75 eV, has promising application in optoelectronic device, sensor and photocatalysis [11, 12, 13, 14, 15, 16]. In particular, In2O3 shows superior effect in photocatalytic degradation of dyes. Liu and co-workers reported In2O3 nanocubes with high photocatalytic activity for degenerating eosin B under UV irradiation [17]. Cao et al. synthesized hollow In2O3 nanocrystals with efficient photocatalytic degradation of RhB and MB under UV irradiation [18]. Zhang et al. reported In2O3 porous spheres possessed superior photocatalytic degradation of RhB under UV irradiation [19]. As the morphology of the nanomaterial is one of the key factors that affect the properties, the shape control of nanostructures has been investigated extensively. In recent years, various nano/micro-sized In2O3 with different morphologies have been synthesized [17, 19, 20, 21, 22, 23]. These nanostructures not only create intriguing variety of morphology features, but also provide good blocks for developing high photocatalytic performance.
In this work, we demonstrate a simple strategy for the synthesis of In(BTC)(phen)(H2O) precursor without any additives. Just by modulating the volume ratio of DMF:H2O:C2H5OH while keeping all the other conditions unchanged, a series of In(BTC)(phen)(H2O) nanostructures with different sizes and shapes was obtained. After calcination in air, the as-prepared In(BTC)(phen)(H2O) was subsequently converted to In2O3 nanostructures while their original morphologies were well maintained. Afterwards, photocatalytic performances of the as-obtained In2O3 were investigated and In2O3 nanocrystals exhibited excellent photocatalytic activity for the degradation of RhB molecules under UV light irradiation.
2. Experimental2.1. Sample preparation
Materials and chemicals: In(NO3)3·6H2O, 1, 3, 5-benzenetricarboxylic acid (H3BTC), 1, 10-phenanthroline (phen), N, N-dimethylformamide (DMF) and ethanol (C2H5OH) were obtained from Nanjing Chemical Reagent Co., Ltd. All chemicals are analytically pure and were used as received without further purification.
2.1.1. Synthesis of precursorIn a typical experiment, In(BTC)(phen)(H2O) was prepared by procedures reported previously [24]. In(BTC)(phen)(H2O) nanostructureswere obtained by solvothermal reaction of In(NO3)3·6H2O (18.4mg, 0.045mmol), H3BTC (9.5 mg, 0.045mmol), phen (8.9 mg, 0.045mmol) and 30 mLsolvent ofDMF, H2O and C2H5OHin a 43mL Teflon-lined autoclave, which was sealed and maintained at 130 ℃ for 4 h. The reaction vessel was then removed from the oven and allowed to cool to roomtemperature. The particleswere isolated by centrifugation and washed several times withDMF and C2H5OHand dried in a vacuum at 60 ℃ for 5 h. Anal. Calcd. for C21H13N2O7In: C 48.45, H 2.50, N 5.38%. Found: C 48.30, H 2.18, N 5.64%.
2.1.2. Synthesis of In2O3 nanocrystals
The as-obtained precursor was calcinated in air at 400 ℃ for 1.5 h with a heating rate of 2 ℃ min-1 to produce In2O3 nanostructures.
2.2. MeasurementsPowder X-ray diffraction (PXRD) data were obtained on a Bruker D8 Advance X-ray diffractometer with Cu-Kα radiation (λ = 1.5418 Å ). Scanning electron microscopy (SEM) images were measured on a field emission scanning electron microanalyzer (Hitachi S-4800), employing an accelerating voltage of 5 kV. Transmission electron microscopy (TEM) images were obtained on a JEM-2100 high resolution transmission microscope (HRTEM), employing an accelerating voltage of 200 kV. FT-IR spectra were recorded in the range of 400-4000 cm-1 on a Bruker Vector22 FT-IR spectrophotometer using KBr pellets. Elemental analysis for C, H, and N was performed on a Perkin- Elmer 240C Elemental Analyzer. Thermogravimetric analyses (TGA) were performed on a Mettler-Toledo (TGA/DSC1) thermal analyzer under the nitrogen atmosphere with a heating rate of 10 ℃ min-1. The UV-vis spectra were recorded on a Shimadzu UV-3600 spectrophotometer. Sorption experiments at 77 K were carried out on a Belsorp-max volumetric gas sorption instrument. Surface areas were determined by the BET equation. Prior to measurement of sorption, the samples were activated under vacuum at 100 ℃ for about 10 h.
2.3. Photocatalytic activity testThe photocatalytic activity of the as-obtained crystalline In2O3 nanomaterials was evaluated by photodegradation of rhodamine B (RhB) dye aqueous solution under UV irradiation. A 300WHg arc lamp was used as a light source to provide the UV light at the wavelength of 365 nm. In2O3 (40 mg) was dispersed into rhodamine B aqueous solutions (40 mL) with a concentration of 1 × 10-5 mol L-1 and irradiated by UV light for different time at room temperature.
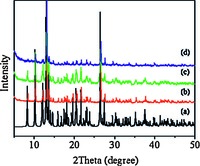
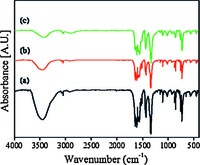
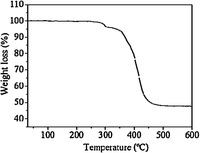
3. Results and discussion 3.1. Preparation and characterizationFig. 1 shows the PXRD patterns of the In(BTC)(phen)(H2O) nanostructures. The data imply the unique crystalline phase, and are in good agreement with the simulated one from the single crystal data [23]. The FT-IR spectral data further support the chemical composition of the In(BTC)(phen)(H2O) nanostructures (Fig. 2). The characteristic bands around 1608 and 1342 cm-1 are the stretching vibrations of νas(-COO-) and νs(-COO-) of the carboxylate in BTC3-. The TGA curve of the In(BTC)(phen)(H2O) nanostructures displays two main steps of weight loss (Fig. 3). The first weight loss from 270 ℃ to 310 ℃ is attributed to the loss of coordinated water molecules and the second one is due to the decomposition of the In(BTC)(phen) framework from 340 ℃ to 470 ℃.
|
Download:
|
| Fig. 1.XRD patterns of simulated In(BTC)(phen)(H2O) and the as-obtained precursors with different volume ratio of DMF:H2O:C2H5OH: (a) simulated, (b) 1:1:1, (c) 2:1:1 and (d) 4:1:1. | |
|
Download:
|
| Fig. 2.FT-IR spectra of the as-obtained precursors with different volume ratio of DMF:H2O:C2H5OH: (a) 1:1:1, (b) 2:1:1 and (c) 4:1:1. | |
|
Download:
|
| Fig. 3.TGA curves of In(BTC)(phen)(H2O) nanocrystals. | |
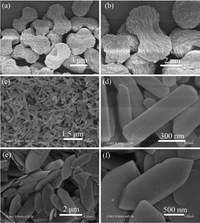
Effectof solventonthe formationof In(BTC)(phen)(H2O) particles was investigated, and it was found that the reaction solvent played a crucial role in the formation of particles with different sizes and shapes. Fig. 4 shows field emission SEM images of In(BTC)(phen)(H2O) nanostructures under different volume ratio of DMF:H2O:C2H5OH.When the volume ratio of DMF:H2O:C2H5OH is 1:1:1, the uniform hierarchical straw-sheaf-like architectures made from an abundance of nanorods with diameters of 50 nm and lengths of 100 nm are obtained and the average particle sizes are around 5 mm(Fig. 4a and b). The crystal aggregate was dispersed to the uniform nanorods with average lengths of about 1 mm and 200 nmin major and minor axes, respectively (Fig. 4c and d), when theDMF:H2O:C2H5OHratio increases to 2:1:1.Withfurther increase of the DMF:H2O:C2H5OH ratio to 4:1:1, the elongated hexagonal particles are obtained with average lengths of about 2.5 mm and 600 nminmajor andminor axes, respectively (Fig. 4e and f). In short, with increasing the DMF content in the mixed solvent, hierarchical straw-sheaf-like architectures dispersed to small monodisperse nanorods first, and then those nanorods gradually increased in size and appeared in elongated hexagonal shapes. Apparently, the size of crystal can be easily tuned by the volume ratio ofDMF:H2O:C2H5OH. It indicates that DMF plays an important role during the crystal growth process and affects the crystal nucleation and growth rate, which can be explained by changing the solubility ofmetal salts and ligands in the mixed solvent. Generally speaking, the pattern formed by crystallization is generally affected by the balance of the driving force for the crystallization and the diffusion rate of ions or molecules [25, 26]. When increasing the content of DMF, the solubility of metal salts and ligands is raised, and thus the diffusion rates are accelerated, which decreases the driving force for the crystallisation relative to that for diffusion; thus, the ligands could not link themetal ionsimmediately and thenucleationratebecomes slow, larger size particles were obtained.
|
Download:
|
| Fig. 4.SEM images of the as-obtained precursors with different shapes: (a and b) straw-sheaf-like architectures, (c and d) nanorods and (e and f) elongated hexagons. | |
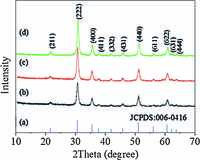
On the basis of the results of TGA, it was found that the proper temperature to obtain In2O3 fromIn(BTC)(phen)(H2O) is 400 ℃. As a result, the thermal decomposition of the corresponding In(BTC)(phen)(H2O) nanocrystals was performed at 400 ℃. After being annealed at 400 ℃ for 1.5 h in air, the as-synthesized In(BTC)(phen)(H2O) nanocrystals were completely converted to phase-pure In2O3. Fig. 5 shows the morphology of the as-obtained products, it can be clearly seen that the original shape can be maintained after calcination. Fig. 5g shows a representative TEM image of the In2O3 nanorods. From high-magnification TEMimages (Fig. 5h), the inter-planar distance of 0.503 nmis in good agreement with the (2 0 0) plane of cubic In2O3. The crystallographicphase of all threesamples is again examined byPXRD(Fig. 6). All the peaks are in good accordance with the standard spectrum of In2O3 (JCPDS 06- 0416), which means that the precursor has been completely transformed into the In2O3 nanostructures.

|
Download:
|
| Fig. 5.SEM images of the as-obtained In2O3 with different shapes: (a and b) straw-sheaf-like architectures, (c and d) nanorods, (e and f) elongated hexagons, (g) TEM and (h) HRTEM of as-prepared In2O3 nanorods. | |
|
Download:
|
| Fig. 6.Standard spectrum of In2O3 (a), XRD patterns of as-obtained In2O3 with different shapes: (b) straw-sheaf-like architectures, (c) nanorods and (d) elongated hexagons. | |
The photocatalytic activities of the as-obtained In2O3 products were investigated by degrading the RhB solution under UV light irradiation. Fig. 7 shows the comparison of photocatalytic activities among the In2O3 products with different morphologies. In the absence of catalysts, the photolysis of RhB under UV light was almost negligible, less than 7% after irradiation for 8 h. In addition, the In2O3 products with hierarchical straw-sheaf-like architectures, nanorods and elongated hexagonal shapes show the degradation efficiencies of 86%, 94%, and 89%, respectively. It is known that the photocatalytic activity of metal oxide particles is closely related to their surface area and pore volume [16, 18, 27]. Therefore, the more effective photocatalytic activity of In2O3 nanorods may be attributed to the larger BET surface area and pore volume, which provides more photocatalytic sites. To investigate the BET surface area of the In2O3 nanostructures with different morphologies, nitrogen sorption measurements were performed. Nitrogen adsorption-desorption isotherms of the asobtained products are shown in Fig. 8. The BET surface areas and pore volumes of the products are 28.3 m2 g-1 and 109.2 mm3 g-1, 33.4 m2 g-1 and 124.3 mm3 g-1, and 29.6 m2 g-1 and 120 mm3 g-1 for hierarchical straw-sheaf-like architectures, nanorods and elongated hexagons, respectively. Therefore, the highest photocatalytic capacity of nanorods may be attributed to the largest BET surface area and pore volume, which is beneficial for the degradation of RhB.

|
Download:
|
| Fig. 7.Normalized degradation curves of RhB solution with the (a) blank test, asobtained In2O3 with different shapes: (b) straw-sheaf-like architectures, (c) nanorods and (d) elongated hexagons. | |

|
Download:
|
| Fig. 8.N2 adsorption-desorption isotherms of the as-obtained In2O3 with different shapes at 77 K: (a) straw-sheaf-like architectures, (b) nanorods and (c) elongated hexagons. | |
In summary, this work explored an effective strategy for tuning the size and morphology of In(BTC)(phen)(H2O) crystals by changing the volume ratio of DMF:H2O:C2H5OH. In the presence of DMF, crystal size and shape can be well tailored upon variation of the volume ratio of DMF:H2O:C2H5OH, resulted in hierarchical straw-sheaf architectures, nanorods and elongated hexagonal crystals with different sizes. After converting the samples into pure cubic In2O3 by thermal annealing, the obtained products show no substantial morphological alternation. The In2O3 samples have been demonstrated to have good photocatalytic properties in the degradation of RhB. Furthermore, In2O3 nanorods have the highest photocatalytic activity, which is attributed to the large BET surface area and pore volume.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 21331002). This work was also supported by a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
| [1] | N.L. Rosi, J. Eckert, M. Eddaoudi, et al., Hydrogen storage in microporous metal-organic frameworks, Science 300(2003) 1127-1129. |
| [2] | T.K. Maji, R. Matsuda, S. Kitagawa, A flexible interpenetrating coordination framework with a bimodal porous functionality, Nat. Mater. 6(2007) 142-148. |
| [3] | H.L. Guo, Y.Z. Zhu, S.S. Wang, et al., Combining coordination modulation with acid-base adjustment for the control over size of metal-organic frameworks, Chem. Mater. 24(2012) 444-450. |
| [4] | K.M.L. Taylor-Pashow, J.D. Rocca, Z.G. Xie, S. Tran, W.B. Lin, Postsynthetic modifications of iron-carboxylate nanoscale metal-organic frameworks for imaging and drug delivery, J. Am. Chem. Soc. 131(2009) 14261-14263. |
| [5] | D. Zacher, O. Shekhah, C. Wö ll, R.A. Fischer, Thin films of metal-organic frameworks, Chem. Soc. Rev. 38(2009) 1418-1429. |
| [6] | F. Gao, H. Pang, S.P. Xu, Q.Y. Lu, Copper-based nanostructures:promising antibacterial agents and photocatalysts, Chem. Commun. (2009) 3571-3573. |
| [7] | L.N. Jin, Q. Liu, W.Y. Sun, Room temperature solution-phase synthesis of flowerlike nanostructures of[Ni3(BTC)2·12H2O] and their conversion to porous NiO, Chin. Chem. Lett. 24(2013) 663-667. |
| [8] | S.J. Yang, T. Kim, J.H. Im, et al., MOF-derived hierarchically porous carbon with exceptional porosity and hydrogen storage capacity, Chem. Mater. 24(2012) 464-470. |
| [9] | J.M. Yang, W. Zhang, Q. Liu, W.Y. Sun, Porous ZnO and ZnO-NiO composite nano/microspheres:synthesis, catalytic and biosensor properties, RSC Adv. 4(2014) 51098-51104. |
| [10] | Y. Ma, Y.H. Ni, F. Guo, N.N. Xiang, Flowerlike copper(II)-based coordination polymers particles:rapid room-temperature fabrication, influencing factors, and transformation toward CuO microstructures with good catalytic activity for the reduction of 4-nitrophenol, Cryst. Growth Des. 15(2015) 2243-2252. |
| [11] | S. Parthiban, K. Ramamurthi, E. Elangovan, R. Martins, E. Fortunato, Spray deposited molybdenum doped indium oxide thin films with high near infrared transparency and carrier mobility, Appl. Phys. Lett. 94(2009) 212101. |
| [12] | F. Liu, M.Q. Bao, K.L. Wang, et al., One-dimensional transport of In2O3 nanowires, Appl. Phys. Lett. 86(2005) 213101-213103. |
| [13] | B.X. Li, Y. Xie, M. Jing, et al., In2O3 hollow microspheres:synthesis from designed In(OH)3 precursors and applications in gas sensors and photocatalysis, Langmuir 22(2006) 9380-9385. |
| [14] | C. Li, M. Curreli, H. Lin, et al., Complementary detection of prostate-specific antigen using In2O3 nanowires and carbon nanotubes, J. Am. Chem. Soc. 127(2005) 12484-12485. |
| [15] | J.B. Mu, C.L. Shao, Z.C. Guo, et al., In2O3 nanocubes/carbon nanofibers heterostructures with high visible light photocatalytic activity, J. Mater. Chem. 22(2012) 1786-1793. |
| [16] | L.M. Lang, D. Wu, Z. Xu, Controllable fabrication of TiO2 1D-nano/micro structures:solid, hollow, and tube-in-tube fibers by electrospinning and the photocatalytic performance, Chem. Eur. J. 18(2012) 10661-10668. |
| [17] | S.Q. Guo, X. Zhang, Z.W. Hao, et al., In2O3 cubes:synthesis, characterization and photocatalytic properties, RSC Adv. 4(2014) 31353-31361. |
| [18] | J.F. Yin, H.Q. Cao, Synthesis and photocatalytic activity of single-crystalline hollow rh-In2O3 nanocrystals, Inorg. Chem. 51(2012) 6529-6536. |
| [19] | B.L. Tao, Y. Zhang, D.Z. Han, Y.P. Li, Z.F. Yan, Synthesis of corundum-type In2O3 porous spheres and their photocatalytic properties, J. Mater. Chem., A 2(2014) 5455-5461. |
| [20] | J.H. Huang, L. Gao, Anisotropic growth of In(OH)3 nanocubes to nanorods and nanosheets via a solution-based seed method, Cryst. Growth Des. 6(2006) 1528-1532. |
| [21] | D.Yu, S.H.Yu, S. Zhang, et al.,Metastable hexagonal In2O3 nanofibers templated from InOOH nanofibers under ambient pressure, Adv. Funct. Mater. 13(2003) 497-501. |
| [22] | L.Y. Chen, Z.X. Wang, Z.D. Zhang, Corundum-type tubular and rod-like In2O3 nanocrystals:synthesis from designed InOOH and application in photocatalysis, New J. Chem. 33(2009) 1109-1115. |
| [23] | L.Y. Chen, Y.G. Zhang, W.Z. Wang, Z.D. Zhang, Tunable synthesis of various hierarchical structures of In(OH)3 and In2O3 assembled by nanocubes, Eur. J. Inorg. Chem. 2008(2008) 1445-1451. |
| [24] | B. Gómez-Lor, E. Gutiérrez-Puebla, M. Iglesias, et al., Novel 2D and 3D Indium metal-organic frameworks:topology and catalytic properties, Chem. Mater. 17(2005) 2568-2573. |
| [25] | H. Imai, Y. Oaki, Bioinspired hierarchical crystals, MRS Bull. 35(2010) 138-144. |
| [26] | J.M. Yang, Q. Liu, Y.S. Kang, W.Y. Sun, Controlled growth and gas sorption properties of IRMOF-3 nano/microcrystals, Dalton Trans. 43(2014) 16707-16712. |
| [27] | J.B. Mu, B. Chen, M.Y. Zhang, et al., Enhancement of the visible-light photocatalytic activity of In2O3-TiO2 nanofiber heteroarchitectures, ACS Appl. Mater. Interfaces 4(2012) 424-430. |
 2016, Vol.27
2016, Vol.27 


