In the last few years, the removal of dyes from industrial effluents has been given much more attention, not only because of their potential toxicity, but also due to their damagining nature to environment [1, 2, 3, 4]. In addition, the presence of these dyes even at a very low concentrations can pollute a large water body, which not only affects aesthetic nature but also reduces sunlight penetration and photosynthesis processes. They may also cause some adverse effects such as, allergic, dermatitis, skin irritation, cancer and mutations in humans [5, 6, 7]. Therefore, dye removal has been a very important but challenging research area of wastewater treatment.
Nowadays, adsorption of dyes is carried out with superabsorbent materials due to their high water absorption property [8, 9]. Superabsorbent materials have specific functional groups that will increase the water as well as dye adsorption on their surface. Many attempts have been made to develop new superabsorbent hydrogels using various methods such as microemulsion, irradiation and chemical cross-linking [10, 11]. Hydrogels prepared by the microemulsion polymerization method have small particles with high surface area that increase the water and dye absorbency [12, 13]. Microemulsion polymerization has been widely studied due to the fact that it synthesizes particles of controlled size and shape, which can be divided into oil-in-water (direct), water-in-oil (reverse), and water-in-oil-in-water (double) polymerizations. Such nanohydrogels prepared by microemulsion polymerization have excellent properties, which have better water and dye absorption than hydrogels synthesized by simpler methods [14]. Also, the vast difference in swelling behaviors and large amount of dye adsorption was observed in the present studies than our previous research due to the fact that the synthesized hydrogels were nano particles in nature.
Based on the background information mentioned above, to acquire a new hydrogel with excellent properties in nano sized particles, inverse microemulsion polymerization was carried out to synthesize poly(NIPAAm/DAPB/AA) (NIPAAm: N-isopropylacrylamide; DAPB: N, N-diallylpyrrolidinium bromide; AA: Acrylic acid nanohydrogels). Adsorption ability of the nanohydrogel for the removal of dyes namely, Neutral Red (Basic Red 5 (BR-5)), Safranin O (Basic Red 2 (BR-2)) and Indigo Carmine (Acid Blue 74 (AB-74)) from aqueous solutions was investigated. After then, kinetics and isotherms adsorption models, such as pseudo-first order, pseudo-second order, Langmuir, and Freundlich were evaluated to examine the maximum adsorption and mechanism of the sorption process.
2. ExperimentalInverse microemulsion polymerization was employed to synthesize superabsorbent nanohydrogels in which DAPB monomer was used to adjust the ratio of hydrophilic and hydrophobic segments of the nanohydrogel, which also produced the amphoteric nature of nanohydrogel framework for better adsorption. Firstly, in a continuous phase, 0.5 g of aerosol (2-hydroxyethyl sulfosuccinate) sodium salt) (AOT) was added to 5 mL of toluene and the mixture was stirred under dry N2 for 30 min. The temperature of the flask was maintained at 60 ℃ using a temperature controller.
The disperse phase was prepared by dissolving a required amount of NIPAAm with different amount of DAPB and AA. The solution was stirred under N2 till a homogeneous solution was obtained. The disperse phase was then added dropwise into the continuous phase to form W/O microemulsion. A cross-linking agent, ethylene glycol dimethacrylate (EGDMA)was added followed by the addition of 2,2'-azobisisobutyronitrile (AIBN) as a surface active initiator. Total conversion was obtained after 7 h of reaction.
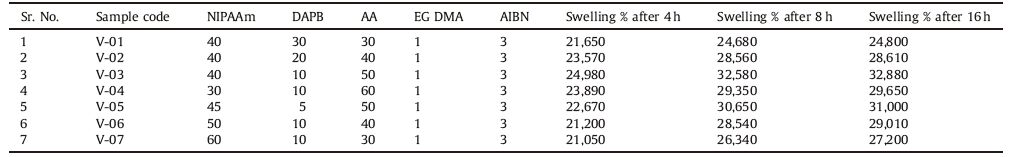
Formed hydrogels were then transferred to a 1 L beaker containing double distilled water and left for 2-3 days by changing water at every 4 h of interval in order to remove the unreacted monomers and other reactants. The swollen gel was dried using acetone in order to confirm the porosity in hydrogel is generated during solvent drying. The processwas repeated till the dry hydrogel was obtained. Finally, the hydrogel was kept in vacuum oven to constant weight. The feed composition and relative swelling percentage of the nanohydrogels (V-01 to V-07) are given in Table 1.
|
|
Table 1 The feed composition and relatively percentage swelling of nanohydrogel. |
Swelling behaviors of poly(NIPAAm/DAPB/AA) nanohydrogels were determined by the gravimetry method at 25 ℃ by adopting a tea-bag [15]. Swelling percentage was calculated by the following equations:


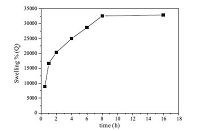
Fig. 1 shows the effect of the contact time on the swelling percentage of the nanohydrogel (V-03). Fast swelling of the nanohydrogels was achieved up to 8 h. After that, increasing the contact time up to 16 h resulted in insignificant swelling. Also, the results were identical for all the synthesized nanohydrogels (Table 1). Therefore, the equilibrium time was chosen as 8 h for further dye adsorption studies.
|
Download:
|
| Fig. 1.The effect of the contact time on swelling percentage of the nanohydrogel. | |
Poly(NIPAAm/DAPB/AA) superabsorbent nanohydrogels (V-01 to V-07) were synthesized using different composition of monomers, surfactant, initiator, and cross-linker. The schematic representation of the synthesis of nanohydrogels is shown in Scheme 1. Sample coded nanohydrogel V-03 shows a high equilibrium swelling percentage after 8 h and has good stability, absorption and mechanical properties than other, so V-03 was utilized for further characterization and dye adsorption studies.

|
Download:
|
| Scheme 1.Reaction scheme of poly(NIPAAm/DAPB/AA) nanohydrogel. | |
It is well known that there are mainly two factors that affect the adsorption of dyes. The first one is electrostatic interactions and the other is hydrophobic interactions. Electrostatic interactions occur between ionizable groups of a hydrogel and the specific charges of a dye molecule, while hydrophobic interactions may involve the aromatic rings and the methyl group in the dye molecules and the methine groups in the nanohydrogel. It is interesting that the synthesized nanohydrogels are amphoteric in nature and therefore, they are capable of adsorbing both cationic and anionic dyes on their surface in high proportion.
The maximum adsorption capacity of nanohydrogel was investigated as a function of time and the results were illustrated in Fig. 2. In an adsorption study, 25 mg of nanohydrogel was treated with 50 mL solution (300 ppm) of each dye at their natural pH value. It was observed that the adsorption of the dyes increased with increasing time and attained almost a constant value at equilibrium after a specific time (8 h). It was also observed that the adsorption of the dyes was fast at the initial stages and became slower near the equilibrium and reached a steady value at equilibrium. After increasing the adsorption time up to 16 h, there was not an intrinsic change in the adsorption of dyes due to the lack of active sites on the nanohydrogel. From this study, it was concluded that the time has significant influence on the adsorption of the dyes [16].

|
Download:
|
| Fig. 2.The effect of contact time on dyes adsorption (initial dye concentration: 300 ppm, adsorption dose: 25 mg). | |
In order to investigate the applicability of the superabsorbent amphoteric nanohydrogel, it was necessary to obtain knowledge on its sorption capacity toward cationic and anionic dyes. This was carried out by equilibrating a fixed amount of nanohydrogel with a series of dye solutions of gradually increased concentrations. Fig. 3 described the adsorption of BR-5, BR-2 and AB-74 on nanohydrogel with different initial dye concentrations. It was observed that adsorption of BR-5 and BR-2 on the nanohydrogel is greater than AB-74 to an almost equal degree. The effect of the initial dye concentration depends on the relationship between the dye concentration and the available active sites on an adsorbent surface. Adsorption increased with the increase in the initial dye concentration due to more activated sites were available. After that, specific change in adsorption was not observed because of active sites required for adsorption of dye molecules will not be available [17].

|
Download:
|
| Fig. 3.The effect of initial dye concentration on dyes adsorption (treatment time: 8 h, adsorption dose: 25 mg). | |
The pH of the solutions plays a significant role in affecting the maximum adsorption, particularly when the adsorbent and adsorbate are both charged species. In the current study, the adsorbate, cationic and anionic dyes, and the adsorbent poly(- NIPAAm/DAPB/AA) all contain ionizable carboxylic acid groups as well as quaternary positive charges along the polymer chains.
Effect of pH (2-12) was investigated in the batch process and the results are presented in Fig. 4. For the experiment, all the dye solutions were prepared with the concentration at 200 ppm for AB-74 and 300 ppm for BR-5 and BR-2. To 50 mL solutions of each dye was added nanohydrogel (25 mg) and the mixture was stirred for 8 h at 100-150 rpm at room temperature. Fig. 4 shows that the pH has a profound effect on dye adsorption, i.e. nanohydrogel can absorb maximum amount of dyes at specific pH value. At basic pH, the -COOH groups ionize to -COO- ions and therefore, dye adsorption occurs via the strong ionic interactions between the positive charges of the dye molecule and the negative charges of -COO- groups of the nanohydrogel. Also, interactions between quaternary amine of nanohydrogel chain with -COONa and -SO3Na groups of the dye molecules resulted in the anionic dye adsorption. At acidic pH, ionic interaction is comparatively weak due to partial positive charge of the -COO- group of nanohydrogel and also, at higher pH the diffusion of H+ ions occurs in the dye molecules [18].

|
Download:
|
| Fig. 4.The effect of pH on dyes adsorption (initial dye concentration: 300 ppm, treatment time: 8 h, adsorption dose: 25 mg). | |
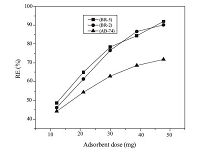
For the adsorption study, the adsorbent doses were increased from 10 mg to 50 mg for dyes concentration of 300 ppm for 8 h at ambient temperature and results are shown in Fig. 5. It is well known that the adsorption occurs on the activated sites of the dye molecules. The increase in adsorbent dosage leads to a decrease in the adsorption capacity of dye solutions. At first, increase in adsorption was due to the active sites remaining for adsorption and after that, saturation was observed due to the lack of active sites on nanohydrogel chain.
|
Download:
|
| Fig. 5.The effect of adsorption dose on dyes adsorption (initial dye concentration: 300 ppm, treatment time: 8 h). | |
Dyes adsorbed on nanohydrogel have been desorbed in a batch wise mode in two steps:
(1) 0.2 mol/L HCl, in a ratio of 50 mL/0.05 g dry dyes adsorbed nanohydrogel have been added in 120 min in this step, followed by washing at neutral pH;
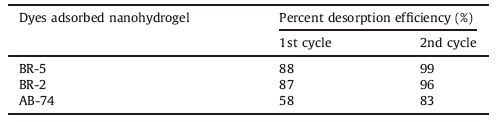
(2) 0.2 mol/L NaOH, the same ratio like HCl, was added to the remaining dyes adsorbed solution for 2 h. Repeated the process until the maximum removal of dyes from nanohydrogel. After washing at neutral pH, the gels have been ready for a new cycle of sorption/desorption process [19]. Also, throughout the process negligible change in the weight of nanohydrogel was observed. The detail desorption process for dyes adsorbed nanohydrogel is given in Table 2.
|
|
Table 2 The feed composition and relatively percentage swelling of nanohydrogel. |
Desorption studies can help elucidating the mechanism of an adsorption process. In the present case, strong acids or bases, such as HCl or NaOH can desorb the dyes adsorbed on nanohydrogel in high percent. Therefore, it can be said that maximum adsorption was achieved by ion exchange or electrostatic attractions.
4. ConclusionsIn this study, we have synthesized a new type of superabsorbent poly(NIPAAm/DAPB/AA) amphoteric nanohydrogels by the inverse microemulsion polymerization method. The success of the synthesis was confirmed by different analytical techniques such as FT-IR, DSC, TGA, SEM and TEM. The particle size distribution of the nanohydrogel ranged in between 30 nm and 40 nm. Clear evidence for the adsorption of dyes on the nanohydrogel surface was achieved from SEM images. Surface area, 19.028 m2/g and pore volume, 0.008 cm3/g of nanohydrogel were determined by the BET and BJH analyses. The LCST of nanohydrogel was greater due to more hydrophilic groups in the polymer network. Synthesized nanohydrogel was able to remove a high percentage of both cationic and anionic dyes due to its amphoteric nature. Effect of pH on adsorption of dyes was marvelous. A Maximum removal efficiency of BR-5 in 300 ppm is 94.48%, BR-2 in 300 ppm is 91.71%, and AB-74 in 200 ppm is 69.30%. The adsorption data were analyzed by the kinetics and isotherms adsorption models, and the results showed that pseudo-second order kinetic isotherm described the adsorption process of nanohydrogel well. These improvements in the adsorption behaviors encourage the efforts for the nanohydrogel to be used in wastewater treatment.
AcknowledgmentsThe authors gratefully acknowledge to Head, Department of Chemistry at Sardar Patel University for providing research facilities. Appreciation is expressed for the studies in the Sophisticated Instrument Center for Applied Research and Testing [SICART], Vallabh Vidyanagar for FT-IR, DSC, TGA, SEM and TEM analysis. Also, appreciation is expressed for Heubach Colour PVT. LTD., Ankleshwar, for free of cost BET and BJH analysis. Financial support for this work was provided by University Grants Commission, New Delhi (No. F.39-685/2010(SR)), to whom researches are gratefully acknowledged.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2015.12.015.
| [1] | L.L. Fan, Y. Zhang, X.J. Li, et al., Removal of alizarin red from water environment using magnetic chitosan with Alizarin Red as imprinted molecules, Colloids Surf., B:Biointerfaces 91(2012) 250-257. |
| [2] | (Ⅰ). Ali, M. Asim, T.A. Khan, Low cost adsorbents for the removal of organic pollutants from wastewater, J. Environ. Manage. 113(2012) 170-183. |
| [3] | M.A.M. Salleh, D.K. Mahmoud, W.A.W.A. Karim, A. (Ⅰ)dris, Cationic and anionic dye adsorption by agricultural solid wastes:a comprehensive review, Desalination 280(2011) 1-13. |
| [4] | Y.J. Tang, X. Wang, L.H. Zhu, et al., Removal of methyl orange from aqueous solutions with poly(acrylic acid-co-acrylamide) superabsorbent resin, Polym. Bull. 70(2013) 905-918. |
| [5] | H. Yao, X.M. You, Q. Lin, et al., Multi-stimuli responsive metal-organic gel of benzimidazol-based ligands with lead nitrate and their use in removal of dyes from waste-water, Chin. Chem. Lett. 24(2013) 703-706. |
| [6] | S. Gokturk, S. Kaluc, Removal of selected organic compounds in aqueous solutions by activated carbon, J. Environ. Sci. Technol. 1(2008) 111-123. |
| [7] | J. Zhang, Q.Q. Shi, C.L. Zhang, et al., Adsorption of Neutral Red onto Mn-impregnated activated carbons prepared from Typha orientalis, Bioresour. Technol. 99(2008) 8974-8980. |
| [8] | E. Karadağ, O.B. Üzüm, A study on water and dye sorption capacities of novel ternary acrylamide/sodium acrylate/PEG semi (Ⅰ)PN hydrogels, Polym. Bull. 68(2012) 1357-1368. |
| [9] | E. Karadağ, B. Hasgül, S. Kundakci, O.B. Uzum, et al., Montmorillonite loaded highly swollen AAm/AMPS hydrogels and semi-(Ⅰ)PNs with PEG as a novel composite polymeric sorbent for water and dye sorption, Polym.Plast. Technol. Eng. 53(2014) 1259-1271. |
| [10] | J.J. Wang, F. Liu, Enhanced adsorption of heavy metal ions onto simultaneous interpenetrating polymer network hydrogels synthesized by UV irradiation, Polym. Bull. 70(2013) 1415-1430. |
| [11] | Y.N. Patel, M.P. Patel, A new fast swelling poly[DAPB-co-DMAAm-co-AASS] superabsorbent hydrogel for removal of anionic dyes from water, Chin. Chem. Lett. 24(2013) 1005-1007. |
| [12] | F.M.Pavel,Microemulsionpolymerization, J. DispersionSci.Technol.25(2004)1-16. |
| [13] | N. Sahiner, Hydrogel nanonetworks with functional core-shell structure, Eur. Polym. J. 43(2007) 1709-1717. |
| [14] | P.V. Dadhaniya, M.P. Patel, R.G. Patel, Removal of anionic dyes from aqueous solution using poly[N-vinyl pyrrolidone/2-(methacryloyloxyethyl) trimethyl ammonium chloride] superswelling hydrogels, Polym. Bull. 58(2007) 359-369. |
| [15] | M. Kaplan, H. Kasgoz, Hydrogel nanocomposite sorbents for removal of basic dyes, Polym. Bull. 67(2011) 1153-1168. |
| [16] | P.V. Dadhaniya, M.P. Patel, R.G. Patel, Swelling and dye adsorption study of novel superswelling[Acrylamide/N-vinylpyrrolidone/3(2-hydroxyethyl carbamoyl) acrylic acid] hydrogels, Polym. Bull. 57(2006) 21-31. |
| [17] | V.P. Mahida, M.P. Patel, Synthesis of new superabsorbent poly(N(Ⅰ)PAAm/AA/Nallylisatin) nanohydrogel for effective removal of As(V) and Cd(Ⅱ) toxic metal ions, Chin. Chem. Lett. 25(2014) 601-604. |
| [18] | H. Wang, X.Z. Yuan, Z.B. Wu, et al., Removal of basic dye from aqueous solution using Cinnamomum camphora sawdust:kinetics, isotherms, thermodynamics, and mass-transfer processes, Sep. Sci. Technol. 49(2014) 2689-2699. |
| [19] | S.S. Li, X.Z. Kong, X.B. Jiang, X.L. Zhu, A novel and simple pathway to synthesis of porous polyurea absorbent and its tests on dye adsorption and desorption, Chin. Chem. Lett. 24(2013) 287-290. |
 2016, Vol.27
2016, Vol.27