Contamination of natural waters is considered one of the most serious threats to human beings. The combined technology involving use of photocatalytic (PC) and electrochemical (EC) processes for the removal of harmful organic compounds in wastewater has developed rapidly in recent years [1, 2]. Photoelectrocatalysis (PEC) represents an advantage over photocatalysis since it applies a potential across a photoanode on which the catalyst is supported. This configuration allows for a more effective separation of the charges generated (e-/h+) in the process, thus increasing the lifetime of the electron-hole pairs [3]. The actual implementation of PEC technology is critically dependent on the characteristics of the semiconductor electrode materials. Among the various electrode materials studied, TiO2 is the most common candidate for the destruction of organic pollutants due to its high photocatalytic activity, good stability, non-toxicity and low cost. However, its wide band gap (~3.2 eV of anatase) limits the effective application of TiO2 under solar irradiation [4, 5]. Therefore, many composite electrode materials involving TiO2 have been investigated, such as, TiO2/SrTiO3 [6], WO3/TiO2 [7], Ti/TiO2 annotates [1], Bi2Ti2O7 [2], Au-NPs/TiO2 annotate [8] and Crdoped TiO2 annotates [9]. The doping with nonmetal elements in a TiO2 nanostructure has shown encouraging results for the development of visible, light-active photoelectrocatalysts [10, 11, 12]. To achieve pre-concentration of pollutants and resolve the difficult separation of TiO2 particles, immobilization of TiO2 on an adsorbent, or an inert support, to form integrated photocatalytic adsorbents (IPAs) has been recommended [13]. Using IPAs, degradation of pollutants can be achieved by the simultaneous effects of physical adsorption by the adsorbent and photochemical degradation by the immobilized TiO2. To realize this, TiO2 particles are typically dispersed on supports with high surface area such as activated carbon [14, 15], and zeolite [16]
Zeolites consisting of three-dimensional structures of SiO4 and AlO4 tetrahedrally linked by oxygen atoms to form a cage-structure possessing good adsorption abilities, uniform channels and regular pores. Thereby, TiO2 can be facilely supported in the existing channels or surface of the zeolite particles. Recently, the studies involving TiO2/zeolite IPAs have exhibited good photocatalytic performance for the removal of organic compounds such as dyes [17, 18, 19], humicacids [20] and pharmaceutical compounds [21] attributed to the advantages of IPAs. With respect to electrocatalysis, the zeolite modified electrodes (ZME) exhibited some good features for the fast, easy and economical electrooxidation of formaldehyde [22]. Furthermore, ZME also have been employed in the oxidation of ascorbic and uric acid, cysteine, methanol and ethanol and so on [23, 24, 25]. Zeolite membranes, as the intergrown material of zeolite crystals, have potential advantages in many applications, such as electrocatalysts and an electrolyte membrane for fuel cells [13] and appears promising to be developed as a novel integrated photocatalytic adsorbent for the removal of organic compounds. However, to date, the study of electrode material based on zeolite membrane-supported N-doped TiO2 used in photoelectrocatalysis has never been reported. This work prepared a novel photoelectrocatalytic electrode material based on N-doped TiO2-coated NaY zeolite membrane, and studied its photoelectrocatalytic performance for phenol degradation.
2. ExperimentalPreparation of zeolite membrane-based electrode material: The porous stainless steel (stn stl) disc measuring 20 mm in diameter and 2 mm in thickness with a porosity of 75% and an average pore size of 0.2 μm was polished with sandpaper (500 mesh), then treated in an aqueous solution of sodium hydroxide (10 mol L-1) for 12 h, dried at 120 ℃ for 24 h and transferred into a desiccator until further use (refer to our previous work [26] for the preparation of the Y-type zeolite membrane). The precursor of N-doped TiO2 was synthesized using sol-gel method [27] and n-butylamine as the doping N source. The N-doped TiO2-coated NaY zeolite membrane was prepared by using the dip-coating method. One side of the supported NaY zeolite membrane was dipped in the precursor of N-doping solution for 30 s, and the procedure was repeated at least three times. After drying in air at 60 ℃, the substrate was calcined at 500 ℃ for 6 h. After cooling, the resulting N-doped TiO2-coated NaY zeolite membrane was washed several times with deionized water until pH 7 and dried at r.t.
Material characterization: The formation of zeolite membranes was confirmed by X-ray diffraction (XRD) using a Bruker-AXS D8 powder diffractometer with Cu Kα (λ = 0.154 nm) radiation (40 kV and 30 mA). The surface morphology was examined by a scanning electron microscopy (SEM) (S4800II, Hitachi) at an acceleration voltage of 15 kV. The UV-vis light absorption spectrum was obtained on a Varian Cary 5000 spectrophotometer equipped with an integrating sphere assembly. The XPS analysis adopted ESCALAB 250Xi spectrometer (Thermo Scientific).
Measurement of catalytic performance: The photoelectrocatalytic activity of the above prepared electrode material was studied by using probe pollutants-phenol under simulated solar-light radiation flux (Source: xenon lamp, PLS-SXE300, 300 W, 200- 2500 nm in the wavelength) and an electric field of 8 V. Herein, the supported N-doped TiO2-coated NaY zeolite membrane, as electrode material, was used as the anode and the porous stn stl disc was used as cathode. The degradation rate of phenol was calculated by measuring the varied levels of the concentration of phenol before and after reaction via UV-vis spectrophotometer (Lambda 850, Perkin Elmer Co.). The evaluation was performed in 100 mL phenol solution at a concentration of 90 mg/L under magnetic stirring. After the completion of degradation, the Ndoped TiO2/NaY zeolite membrane was regenerated by calcination at 500 ℃. The recycling performance of the composite electrode material was also studied.
3. Results and discussionFig. 1 shows the XRD patterns of the as-synthesized NaY zeolite membrane (a) and the N-doped TiO2/NaY zeolite membrane (b). The resulting as-synthesized NaY membrane displays the standard peak of the FAU-type structure (JCPDF:39-1380) as shown in Fig. 1a, confirming that the as-synthesized NaY zeolite membrane has been successfully grown on the porous stn stl disc. After coated by TiO2 precursor and then calcined, the substrate-supported NaY zeolite membrane appears as the fresh peaks at 2θ = 25.3° and 37.8°, which are attributed to the anatase crystal structure of TiO2. This suggests that the TiO2 particles have been successfully coated on the NaY zeolite membrane. Fig. 2 shows SEM images of zeolite membrane before and after coated by TiO2. It can be observed that the NaY zeolite membrane is of relatively better integrity. In contrast to the magnification images, the NaY zeolite membrane consists of the flower-like intergrown crystals (Fig. 2C), and the surface of the N-doped TiO2/NaY zeolite membrane is fully coated by TiO2 nanoparticles with the size of ca. 20 nm (Fig. 2D).

|
Download:
|
| Fig. 1.XRD patterns of the porous stn stl supported NaY zeolite membrane (a) and N-doped TiO2/NaY zeolite membrane (b). | |

|
Download:
|
| Fig. 2.SEM images of supported NaY zeolite membrane (A and C (magnification)) and N-doped TiO2/NaY zeolite membrane (B and D (magnification)). | |
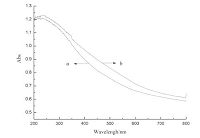
Fig. 3 shows the UV-vis spectra of the N-TiO2 and the N-doped TiO2/NaY zeolite membranes. Besides the typical UV absorption band in the spectra of both samples, a newabsorption band appears in the visible range ofbetween400 nmand 600 nm.This verifies that there is an existing N-Ti banding in N-TiO2. Compared to the Ndoped TiO2 without the supporting NaY zeolite membrane, the visible absorption band of the N-doped TiO2/NaY zeolitemembrane take place as a distinct red-shift. It is likely to be ascribed to the effect of the nano-pore cavity of porous zeolite. Since a part of N-TiO2 nanoparticles are encapsulated in the nano-pore cavity by the electrostatic field, they can produce a nano spectrum-effect under light irradiation [28]. Fig. 4 shows the survey and N 1s XPS spectra of N-doped TiO2/NaY zeolite membrane. In addition to a stronger Ti 2p peak, there appears a weak N 1s peak in the survey XPS spectra (Fig. 4A).Most importantly, along with the N 1s spectra exists the β- N 1s peak, which is considered evidence of the formation of the Ti-N bond [29, 30]. These results verify that the N element has been successfully doped into the crystal structure of TiO2 and suggests that the N-doped TiO2/NaY zeolite membrane is of a visible-light photocatalytic activity.
|
Download:
|
| Fig. 3.UV-vis spectra of N-TiO2 (a) and N-doped TiO2/NaY zeolite membrane (b). | |

|
Download:
|
| Fig. 4.The survey XPS spectra (A) and N 1s XPS spectra (B) of N-doped TiO2/NaY zeolite membrane. | |
Fig. 5 shows the comparison of catalytic activity (A) and recycling performance (B) of the N-doped TiO2/NaY zeolite membrane in 90 mg/L phenol solution under different catalysis patterns. For the electrocatalysis pattern, the degradation of phenol on N-doped TiO2/NaY zeolite membrane shows a lower level. However, the catalytic activity of the N-doped TiO2/NaY zeolite membrane is obviously increased in the photocatalysis pattern under simulated solar light. The degradation rate elevates about two times than that of electrocatalysis pattern after an irradiation time of 60 min, assumed to be due to the effect of Ndoped TiO2. Under the photoelectrocatalysis pattern, the N-doped TiO2/NaY zeolite membrane for the degradation of phenol exhibits the best catalytic activity, degrading phenol of 80% and 89% after 90 min and 180 min of solar-light irradiation, respectively. Interestingly, this is about 10%-20% higher than the sum of the eletrocatalysis and photocatalysis patterns and represents an enhanced synergistic effect of the N-doped TiO2 and the NaY zeolite membrane. For verifying the recycling performance of the electrode material of the N-doped TiO2/NaY zeolite membrane, the number of recycling times were also investigated under the photoelectrocatalysis pattern as shown in Fig. 5B. After recycling 5 times, the degradation rate of the N-doped TiO2/NaY zeolite membrane for phenol decreases slightly from 89% to 75%, exhibiting a good recycling performance.

|
Download:
|
| Fig. 5.Catalytic performance and recycling performance of N-doped TiO2/NaY zeolite membrane (A) comparison; (B) reused stability. | |
The N-doped TiO2-coated NaY zeolite membrane as a novel electrode material was presented in this work. Not only it is of a facilely preparing process, but also exhibits good photoelectrocatalysis activity and better recycling performance, which may provide a promising photoelectrocatalytic material for practical applications in the removal of organic compounds.
AcknowledgmentsWe are grateful to supported by the Talent Introduction Fund of Yangzhou University (2012), Jiangsu Province Science and Technology Support Project (No. BE2014613) and Six Big Peak Talent in Jiangsu Province (No. 2014-XCL-013). The authors also acknowledge the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions. The data in this paper originated from the Test Center of Yangzhou University.
| [1] | E.R.A. Ferraz, G.A.R. Oliveira, M.D. Grando, et al., Photoelectrocatalysis based on Ti/TiO2 nanotubes removes toxic properties of the azo dyes Disperse Red 1, Disperse Red 13 and Disperse Orange 1 from aqueous chloride samples, J. Environ. Manage. 124(2013) 108-114. |
| [2] | S. Gupta, V. Subramanian, Encapsulating Bi2Ti2O7(BTO) with Reduced Graphene Oxide (RGO):an effective strategy to enhance photocatalytic and photoelectrocatalytic activity of BTO, ACS Appl. Mater. (Ⅰ)nterfaces 6(2014) 18597-18608. |
| [3] | P.A. Carneiro, M.E. Osugi, C.S. Fugivara, et al., Evaluation of different electrochemical methods on the oxidation and degradation of Reactive Blue 4 in aqueous solution, Chemosphere 59(2005) 431-439. |
| [4] | D.Y. Wu, M.C. Long, Realizing visible-light-induced self-cleaning property of cotton through coating N-TiO2 film and loading Ag(Ⅰ) particles, ACS Appl. Mater. (Ⅰ)nterfaces 3(2011) 4770-4774. |
| [5] | C.X. Lei, Z.D. Feng, H. Zhou, Visible-light-driven photogenerated cathodic protection of stainless steel by liquid-phase-deposited TiO2 films, Electrochim. Acta 68(2012) 134-140. |
| [6] | J.R. Huang, X. Tan, T. Yu, L. Zhao, W.L. Hu, Enhanced photoelectrocatalytic and photoelectrochemical properties by high-reactive TiO2/SrTiO3 hetero-structured nanotubes with dominant {001} facet of anatase TiO2, Electrochim. Acta 146(2014) 278-287. |
| [7] | M. Zhang, C.Z. Yang, W.H. Pu, et al., Liquid phase deposition of WO3/TiO2 heterojunction films with high photoelectrocatalytic activity under visible light irradiation, Electrochim. Acta 148(2014) 180-186. |
| [8] | L. Wu, F. Li, Y.Y. Xu, et al., Plasmon-induced photoelectrocatalytic activity of Au nanoparticles enhanced TiO2 nanotube arrays electrodes for environmental remediation, Appl. Catal., B:Environ. 164(2015) 217-224. |
| [9] | K. Yang, W.H. Pu, Y.B. Tan, et al., Enhanced photoelectrocatalytic activity of Crdoped TiO2 nanotubes modified with polyaniline, Mater. Sci. Semicond. Process. 27(2014) 777-784. |
| [10] | G.S. Wu, J.P. Wang, D.F. Thomas, A.C. Chen, Synthesis of F-doped flower-like TiO2 nanostructures with high photoelectrochemical activity, Langmuir 24(2008) 3503-3509. |
| [11] | N. Lu, X. Quan, J.Y. Li, et al., Fabrication of boron-doped TiO2 nanotube array electrode and investigation of its photoelectrochemical capability, J. Phys. Chem. C 111(2007) 11836-11842. |
| [12] | X.T. Hong, Z.P. Wang, W.M. Cai, et al., Visible-light-activated nanoparticle photocatalyst of iodine-doped titanium dioxide, Chem. Mater. 17(2005) 1548-1552. |
| [13] | K.L. Yeung, W. Han, Zeolites and mesoporous materials in fuel cell applications, Catal. Today 236(2014) 182-205. |
| [14] | A.Y. Shan, T.(Ⅰ).M. Ghazi, S.A. Rashid, (Ⅰ)mmobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis:a review, Appl. Catal. A:Gen. 389(2010) 1-8. |
| [15] | E.C. Su, B.S. Huang, C.C. Liu, M.Y. Wey, Photocatalytic conversion of simulated EDTA wastewater to hydrogen by pH-resistant Pt/TiO2-activated carbon photocatalysts, Renewable Energy 75(2015) 266-271. |
| [16] | S. Basha, C. Barr, D. Keane, et al., On the adsorption/photodegradation of amoxicillin in aqueous solutions by an integrated photocatalytic adsorbent ((Ⅰ)PCA):experimental studies and kinetics analysis, Photochem. Photobiol. Sci. 10(2011) 1014-1022. |
| [17] | C.Y. Kuo, C.Y. Pai, C.C. He, C.J. Lin, C.M. Cheng, Photodegradation of aqueous reactive dye using TiO2/zeolite admixtures in a continuous flow reactor, Water Sci. Technol. 65(2012) 1963-1969. |
| [18] | C. Wang, H.S. Shi, Y. Li, Synthesis and characteristics of natural zeolite supported Fe3+-TiO2 photocatalysts, Appl. Surf. Sci. 257(2011) 6873-6877. |
| [19] | F.F. Li, Y.S. Jiang, L.X. Yu, et al., Surface effect of natural zeolite (clinoptilolite) on the photocatalytic activity of TiO2, Appl. Surf. Sci. 252(2005) 1410-1416. |
| [20] | S. Liu, M. Lim, R. Amal, TiO2-loaded natural zeolite:rapid humic acid adsorption and effective photocatalytic regeneration, Chem. Eng. Sci. 105(2014) 46-52. |
| [21] | D. Kanakaraju, J. Kockler, C.A. Motti, B.D. Glass, M. Oelgemöller, Titanium dioxide/zeolite integrated photocatalytic adsorbents for the degradation of amoxicillin, Appl. Catal., B:Environ. 166-167(2015) 45-55. |
| [22] | M. Abrishamkar, F.B. Kahkeshi, Synthesis and characterization of nano-ZSM-5 zeolite and its application for electrocatalytic oxidation of formaldehyde over modified carbon paste electrode with ion exchanged synthesized zeolite in alkaline media, Microporous Mesoporous Mater. 167(2013) 51-54. |
| [23] | T. Rohani, M. Ali Taher, A new method for electrocatalytic oxidation of ascorbic acid at the Cu(Ⅱ) zeolite-modified electrode, Talanta 78(2009) 743-747. |
| [24] | M. Mazloum Arkadani, Z. Akrami, H. Kazemian, H.R. Zare, Electrocatalytic characteristics of uric acid oxidation at graphite-zeolite-modified electrode doped with iron(Ⅲ), J. Electroanal. Chem. 586(2006) 31-38. |
| [25] | A. Nezamzadeh-Ejhieh, H.S. Hashemi, Voltammetric determination of cysteine using carbon paste electrode modified with Co(Ⅱ)-Y zeolite, Talanta 88(2012) 201-208. |
| [26] | Z.L. Cheng, H.Q. Lin, Z.S. Chao, H.L. Wan, NaY zeolite membrane prepared by using pre-absorbed nanosized NaY zeolite synthesized by microwave heating, Chem. J. Chin. Univ. 24(2003) 1857-1861. |
| [27] | D. Papoulis, S. Komarneni, A. Nikolopoulou, et al., Palygorskite-and halloysite-TiO2 nanocomposites:synthesis and photocatalytic activity, Appl. Clay Sci. 50(2010) 118-124. |
| [28] | Y.X. Jiang, S.G. Sun, S.P. Chen, N. Ding, Enhancement of (Ⅰ)R absorption of CO adsorbed on palladium-loading zeolite thin film electrode, Chem. J. Chin. Univ. 22(2011) 1850-1863. |
| [29] | S. Sakthivel, M. Janczarek, H. Kisch, Visible light activity and photoelectrochemical properties of nitrogen-doped TiO2, J. Phys. Chem. B 108(2004) 19384-19387. |
| [30] | O. Diwald, T.L. Thompson, T. Zubkov, et al., Photochemical activity of nitrogendoped rutile TiO2(110) in visible light, J. Phys. Chem. B 108(2004) 6004-6008. |
 2016, Vol.27
2016, Vol.27 


