b. College of Chemistry and Chemical Engineering, SWPU, Chengdu 610500, China
CO is one of most dangerous gases in human life and industrial processes. It is hard to detect because it is colorless, odorless, and tasteless. CO can easily combine with hemoglobin to form carboxyhemoglobin, which leads to suspending oxygen transport in the respiratory system even at very low concentrations. Exposure to concentrations around 35 ppm can cause headaches, whereas 1000 ppm can result in sudden death [1, 2, 3].
Thus, it is important to monitor CO via gas sensing materials and devices for early prevention. Many researchers have recently reported the detection of CO using various materials, such as Aldoped ZnO, SnO2, Co3O4, and so on [4, 5, 6]. However, these CO sensors operate at high temperatures, which is not adequately safe for detecting unknown concentration of CO at high temperature derived from explosion limit (12.5%-74%).
Zinc hydroxystannate (ZnSn(OH)6, ZHS), due to its cost and safety, has shown potential across a wide range of applications, namely flame and smoke retardants, gas sensing, and photocatalysts [7, 8, 9]. Among them, one of the most promising applications is in gas sensing. To the best of our knowledge, there are few papers reporting the CO sensing properties of cubic ZHS via QCM [10, 11] at room temperature in dry atmosphere. In this letter, we present a simple and economical hydrothermal method for the production of cubic ZHS on a large scale. The results show that cubic ZHS exhibits excellent CO sensing properties with swift response-recovery, good linearity, and high selectivity to a wide concentration range from 25 ppm to 10, 000 ppm.
2. ExperimentalIn a typical synthesis procedure, 1 mmol of analytical grade zinc acetate (Zn(CH3COO)2-2H2O) was dissolved completely into 40 mL of deionized water at room temperature. Then 1 mmol (SnCl4-5H2O) was added into the Zn(CH3COO)2-2H2O solution. After that, 10 mmol sodium hydroxide (NaOH) was added to the above solution under magnetic stirring for 30 min at room temperature. The homogeneous solution was loaded into a Teflon-lined stainless steel autoclave with a capacity of 50 mL, maintained at 80 ℃ for 20 h, then naturally cooled to room temperature. The crude product was washed with absolute ethanol and double distilled water three times, then suspended as the final product for further characterization and gas sensing testing. The crystal structure of the as-obtained product was characterized by X-ray powder diffraction (XRD, X’Pert PRO) using Cu Kα radiation (λ = 0.1548 nm). The morphology was characterized by scanning electron microscope (SEM, EVO MA15). The gas-sensing properties of ZHS were investigated using a QCM sensor system at room temperature in dry atmosphere.
In detail, ZHS based CO sensor was prepared by the following steps: gold coated QCM was cleaned with ethanol for 20 min. Then the QCM was rinsed in deionized water and dried at room temperature in dry nitrogen atmosphere. The suspension was dropped onto the QCM and the coating was dried in a furnace at 80 ℃ for 1 h. Fig. 1 shows two channel flow system was employed for detecting QCM signals under typical CO concentrations in dry atmosphere to research the adsorption and desorption process. Two mass controller (MFC) valves were used to dilute CO concentrations by N2. The MFC system between 0 and 500 sccm was used to change the ratio of CO/N2 flow in the testing chamber to obtain the desired active gas concentration.

|
Download:
|
| Fig. 1.The QCM measurement for CO gas sensing test. | |
Crystal structures of as-obtained products are investigated by X-ray diffraction (XRD) patterns. Fig. 2a shows that all of the diffraction peaks can be perfectly indexed to the standard ZHS with the perovskite structure (JCPDS No. 20-1455), affirming that the assynthesized sample has a typical FCC crystal structure. The sharp and strong intensity diffraction peaks of ZHS suggest the phase of ZHS is well crystallized [12]. The morphology in Fig. 2(b) characterized by scanning electron microscopy (SEM) shows that a large number of cubic crystals with side lengths of approximately 210 nm were uniformly distributed on surface of the substrate. Meanwhile, SEM image shows the detailed morphological characteristics of cubic ZHS with some nano-pores and sharp corners.

|
Download:
|
| Fig. 2.(a) XRD pattern of the sample and standard XRD pattern of ZHS (JCPDS 20-1455). (b) Typical SEM image of the synthesized ZHS. | |
Fig. 3a describes the response and recovery curves of the QCM sensor based on ZHS and the curves shows a stable, reproducible behavior for the sensor. The sensor was sequentially exposed to various concentrations of CO from 25 ppm to 10, 000 ppm at room temperature in dry atmosphere. The adsorption and desorption frequency of the sensor was observed to change immediately as soon as the sensing materials were exposed to CO gas. The results exhibited swift response and recovery process. Delta F was successfully used to detect the CO with high sensitivity.

|
Download:
|
| Fig. 3.(a) Response and recovery curves of QCM sensor. (b) Delta F (left) and the dependence of the Delta F of sensor (right). | |
Fig. 3b shows the Delta F (left) and dependence of the Delta F of the sensor (right) as a function of CO concentration from 25 ppm to 10, 000 ppm. Delta F increased regularly with increasing CO concentration. Meanwhile, Delta F changes swifter below 500 ppm than the concentration above 1250 ppm, which means that the adsorption process tend to saturation in ultra-high CO concentration. An obvious decrease in the slope was observed from 1250 ppm to 10, 000 ppm; this also resulted from the adsorption tending to saturation. According to the BET adsorption model [13, 14], adsorbed CO onto ZHS surface was considered to be a multi-molecular layer adsorption and the amount of the adsorbed CO is not linear. Moreover, the linear fitting of log(-Delta F) to CO concentration shows good linearity in low (below 500 ppm) and high (above 1250 ppm) concentration ranges, which also suggested that the QCM sensor fabricated by cubic ZHS structures is very suitable to detect CO gas with various concentrations.
Selectivity is an important factor in gas sensors [15, 16, 17, 18]. Thus, we present the response of ZHS coated QCM sensors to various types of gases in Fig. 4, including n-hexane, ether, acetone, ethyl acetate, CH3CH2OH, and CO (25 ppm) to test gas sensitivity, where concentration of five volatile organic compounds are 100 ppm. The sensor based on ZHS film showed high selectivity to CO gas. The highest response about Delta F was about 124 Hz, while the responses to other gases were no greater than 25 Hz. Adsorption energy may help understand selectivity. According to Ou’s report [19], we can predict that the superior selective CO gas response of ZHD originates from its unique physical surface affinity to the gas molecules. Therefore, these results suggest that interference effects from n-hexane, ether, acetone, ethyl acetate, and CH3CH2OH could be negligible during detection of ppm-level CO concentration.
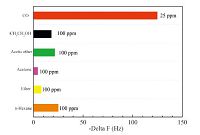
|
Download:
|
| Fig. 4.The frequency response of ZHS coated QCM to various gases. | |
This study researched the CO sensing properties of cubic ZHS that was prepared by hydrothermal method. The microstructure of ZHS tends to be perfect and uniform cubic structures with side length of 210 nm. Meanwhile, the results show that the sensor based on cubic ZnSn(OH)6 film has a swift response and recovery to CO with excellent linearity and high selectivity. This cubic ZnSn(OH)6 is a competitive material for CO testing in a wide range concentrations. This work indicates that ZHS can be used as the CO sensing material for the application of QCM sensors at room temperature.
AcknowledgmentsThisworkwas financially supportedby theKey Project of Sichuan provincial Education office (No. 13ZA0183), Applied Basic Research Programs of Sichuan Provincial Science and Technology Office (No. 2014JY0059), Foundation of Youth Science and Technology Innovation Team of Sichuan Province (No. 2015TD0007) and Programfor NewCentury Excellent Talents in University ofMinistry of Education of China (No. NCET-13-0983).
| [1] | D.D. Trung, N.D. Hoy, P.V. Tong, et al., Effective decoration of Pd nanoparticles on the surface of SnO2 nanowires for enhancement of CO gas-sensing performance, J. Hazard. Mater. 265(2014) 124-132. |
| [2] | C. Özbek, S. Okurb, Ö. Mermer, et al., Effect of Fe doping on the CO gas sensing of functional calixarene molecules measured with quartz crystal microbalance technique, Sens. Actuators B 215(2015) 464-470. |
| [3] | B.R. Sathe, M.S. Risbud, S. Patil, et al., Highly sensitive nanostructured platinum electrocatalysts for CO oxidation:implications for CO sensing and fuel cell performance, Sens. Actuators A 138(2007) 376-383. |
| [4] | S. Vetter, S. Haffer, T. Wagner, M. Tiemann, Nanostructured Co3O4 as a CO gas sensor:temperature-dependent behavior, Sens. Actuators B 206(2015) 133-138. |
| [5] | M. Hjiri, L. El Mir, S.G. Leonardi, et al., Al-doped ZnO for highly sensitive CO gas sensors, Sens. Actuators B 196(2014) 413-420. |
| [6] | T. Yanagimoto, Y.T. Yu, K. Kaneko, Microstructure and CO gas sensing property of Au/SnO2 core-shell structure nanoparticles synthesized by precipitation method and microwave-assisted hydrothermal synthesis method, Sens. Actuators B 166-167(2012) 31-35. |
| [7] | C.Y. Chen, X.Z. Zheng, J. Yang, M.D. Wei, The ZnSn(OH)6 nanocube-graphene composites as an anode material for Li-ion batteries, Phys. Chem. Chem. Phys. 16(2014) 20073-20078. |
| [8] | W.H. Feng, Z.X. Pei, Z.B. Fang, et al., A novel high-photoactivity quaternary ZnSn(OH)6-graphene composite evolved from a 3D multilayer structure via a facile and green proton-mediated self-assembly method, J. Mater. Chem. A 2(2014) 7802-7811. |
| [9] | L.X. Han, J. Liu, Z.Q. Wang, et al., Shape-controlled synthesis of ZnSn(OH)6 crystallites and their HCHO-sensing properties, CrystEngCommun 14(2012) 3380-3386. |
| [10] | G. Sauerbrey, Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung, Z. Phys. 155(1959) 206-222. |
| [11] | J. Xie, H. Wang, M. Duan, QCM chemical sensor based on ZnO colloid spheres for the alcohols, Sens. Actuators B 203(2014) 239-244. |
| [12] | X.L. Fu, D.W. Huang, Y. Qin, et al., Effects of preparation method on the microstructure and photocatalytic performance of ZnSn(OH)6, Appl. Catal. B 148-149(2014) 532-542. |
| [13] | A.K. Ladavos, A.P. Katsoulidis, A. (Ⅰ)osifidis, K.S. Triantafyllidis, T.J. Pinnavaia, P.J. Pomonis, The BET equation, the inflection points of N2 adsorption isotherms and the estimation of specific surface area of porous solids, Micropor. Mesopor. Mater. 151(2012) 126-133. |
| [14] | V.P.J. Chung, M.C. Yip, W. Fang, Resorcinol-formaldehyde aerogels for CMOSMEMS capacitive humidity sensor, Sens. Actuators B 214(2015) 181-188. |
| [15] | S.H. Wang, C.Y. Shen, H.M. Huang, Y.C. Shih, Rayleigh surface acoustic wave sensor for ppb-level nitric oxide gas sensing, Sens. Actuators A 216(2014) 237-242. |
| [16] | X.M. Zhou, W.Y. Fu, H.B. Yang, et al., Novel SnO2 hierarchical nanostructures:synthesis and their gas sensing properties, Mater. Lett. 90(2013) 53-55. |
| [17] | T. Sathitwitayakul, M.V. Kuznetsov, (Ⅰ).P. Parkin, R. Binions, The gas sensing properties of some complex metal oxides prepared by self-propagating hightemperature synthesis, Mater. Lett. 75(2012) 36-38. |
| [18] | W. Zheng, Z.Y. Li, H.N. Zhang, et al., Electrospinning route for α-Fe2O3 ceramic nanofibers and their gas sensing properties, Mater. Res. Bull. 44(2009) 1432-1436. |
| [19] | J.Z. Ou, W.Y. Ge, B. Carey, et al., Physisorption-based charge transfer in twodimensional SnS2 for selective and reversible NO2 gas sensing, ACS Nano 9(2015) 10313-10323. |
 2016, Vol.27
2016, Vol.27 


