The Suzuki and Heck cross-coupling reactions are very important in the synthesis of natural products and fine chemicals [1, 2, 3]. They have been widely applied to diverse areas such as pharmaceuticals, biologically active molecules, and material science, where they provide a powerful and straightforward method for C-C bond formation [4, 5, 6]. To date, many metallic catalysts have been widely studied for Suzuki and Heck reactions. Among the metallic catalysts, palladium-based catalysts have become a hot topic because of their outstanding performance in carbon-carbon cross-coupling [7, 8, 9, 10]. The original Suzuki and Heck reactions was catalyzed by a homogeneous palladium catalyst, which made its separation and recovery tedious, if not impossible, and might result in unacceptable palladium contamination of the products. In order to avoid these disadvantages, heterogeneous catalysts have received extensive attention [11]. As a result, the load type catalyst was prepared, and there are a lot of materials that can be used as the catalyst carrier.
Pd nanoparticles can be supported by a number of materials such as carbon materials [12, 13], polymers [14, 15], and inorganic materials [16, 17, 18]. Carbon materials, including carbon black, carbon nanofibers (CNFs), graphene and other derivatives, have all been used as catalyst carriers [19]. Among these carbon materials, carbon nanofibers (CNFs) have recently attracted considerable attention due to their unique structure, which has a high surface area, a locally conjugated aromatic system, and high chemical and thermal stability [20, 21].
Recently, CNFs have been widely applied as solid supports for catalysts [22, 23]. The CNFs supported catalysts can be simply and efficiently separated from the reaction system by simple filtration, thus avoiding the cumbersome operating process [24]. Peng et al. [25] prepared Pd nano-network structures supported on electrospun carbon nanofibers (Pd-NNSs-ECNFs) as a catalyst for Suzuki coupling reactions, and satisfactory results were obtained in these studies. The obtained Pd-NNSs-ECNFs catalyst showed good activity, high efficiency, and reusability as heterogeneous catalysts for Suzuki coupling under environmentally friendly solvents.
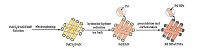
From this point of view, the aim of the present work is to prepare a Pd NPs/CNFs catalyst, which is described in Scheme 1. The PdCl2/PAN nanofibers were obtained by electrospinning. Then, they were reduced by hydrazine hydrate solution in an ice bath, and the Pd/PAN nanofibers were obtained. Finally, the Pd NPs/CNFs were obtained by the high temperature carbonization in a tube furnace. Recently, we have efficiently used Pd supported on CNFs for Suzuki (Scheme 2) and Heck (Scheme 3) reactions. Pd NPs/CNFs exhibited high efficiency, good reusability, and good stability in the Suzuki and Heck reactions.
|
Download:
|
| Scheme 1.Scheme of the preparation of Pd NPs/CNFs. | |

|
Download:
|
| Scheme 2.Suzuki-Miyaura coupling reaction of aryl halides with phenylboronic acid catalyzed by Pd NPs/CNFs. | |

|
Download:
|
| Scheme 3.Mizoroki-Heck cross-coupling reaction of aryl halides with olefinic compounds catalyzed by Pd NPs/CNFs. | |
All reagents were used without further treatment. Polyacrylonitrile (PAN, Mw = 80, 000) was purchased from Kunshan Hong Yu Plastic Co., Ltd. N,N-Dimethylformamide (DMF, C3H7NO, AR, 99.5%) and hydrazine hydrate (H6N2O, 80%) were purchased from Tianjin Fengchuan Chemical Reagent Technology Co. Ltd. Phenylboronic acid (98%), anhydrous ethanol (C2H6O, AR, 99.7%), palladium chloride (PdCl2, AR), methyl acrylate (C4H6O2, CP, 98%), ethyl acrylate (C5H8O2, CP, 98%), iodobenzene (C6H5I, CP, 97%), triethylamine (C6H15N, AR, 99%), and n-butyl acrylate (C7H12O2, CP, 98%) were purchased from Sinopharm Chemical Reagent (China). All other reagents were purchased from Alfa Aesar.
2.2. Preparation of catalystPreparation of PdCl2/PAN/DMF solution: The PdCl2/PAN/DMF solution was prepared according to the previous report from our group [26]. First, 8 wt% PAN/DMF solution was prepared, and then the solution was added into the PdCl2 powder; the molar ratio of the acrylonitrile monomer to the palladium chloride was 30. The PdCl2/PAN/DMF solution was obtained after 12 h of magnetic stirring at room temperature.
Preparation of Pd/PAN nanofibers: The PdCl2/PAN/DMF solution was electrospun to nanofibers by applying a 16 kV voltage to a 15 cm gap between the spinneret and the collector. Then, the PdCl2/PAN nanofibers were reduced by hydrazine hydrate solution in an ice bath. First, hydrazine hydrate solution at a concentration of 2 mol/L was prepared, and then the PdCl2/PAN nanofibers were immersed in it and were reduced in an ice bath environment. Deep brown Pd/PAN nanofibers were obtained and washed several times with distilled water and then dried in air.
Preparation of Pd NPs/CNFs: The Pd NPs/CNFs was obtained by the high temperature carbonization in a tube furnace. The obtained Pd/PAN nanofibers were heated in a tube furnace at 250 ℃ under constant flow of atmosphere for 2 h, the subsequently heated to 350 ℃ under nitrogen and maintained at that temperature for 2 h. Then, it was annealed at 600 ℃ under constant flow of nitrogen for 4 h and cooled to 250 ℃ in nitrogen atmosphere. Finally, it was cooled to room temperature in air.
Characterization of Pd NPs/CNFs catalyst: The UV-vis diffuse reflectance spectrum (DRS) (UV-3600, Shimadzu Corporation) was recorded at wavelengths of 200-700 nm. FT-IR spectra were recorded on a Thermo Nicolet Corporation FT-IR-670. X-ray diffraction (XRD) measurement was carried out using a D/MAX- 2500/PC XRD spectrometer. Field emission scanning electron microscopy (FE-SEM, Quanta 650 FEG Scanning Electron Microscope) was used to characterize the morphology of the catalyst. TEM samples were prepared by drop-casting a dispersion of Pd NPs/CNFs onto carbon-coated copper grids and natural drying.
Catalysis performance of Pd NPs/CNFs for Suzuki-Miyaura crosscoupling reaction: In order to verify the activity of the catalyst, the Suzuki-Miyaura cross-coupling reaction was carried out in our experiments. 5mL of deionized water, 5mL of anhydrous ethanol, 1.0 mmol of aryl halides, 1.1 mmol of phenylboronic acid, 1.5 mmol of base, and 0.005 g of Pd NPs/CNFs catalystwere stirred in a parallel reaction tube for 8 h at 80 ℃. After the reaction completed, the mixturewas filtered at roomtemperature. The filtratewas extracted with ethyl acetate three times and the organic phasewas added with anhydrous Na2SO4 to absorb residualwater. Finally, the solutionwas filtered by an organicmembrane. The productswere determined by GC analysis with the area normalization method. (Conversion rate = 100% × [S1/(S1 + S2)], S1 is the area of all the products, S2 is the area of aryl halides after the reaction; selectivity = 100% × [S1/ (S1 + S2)], S1 is the area of principal product, S2 is the area of all byproducts.) The filter cake (Pd NPs/CNFs) was washed with acetone, ethanol, and distilled water three times.
Catalysis performance of Pd NPs/CNFs for Mizoroki-Heck crosscoupling reaction: 10 g of DMF, 2.0 mmol of aryl halides, 2.7 mmol of olefinic compounds, 6 mmol of base, and 0.001 g of Pd NPs/CNFs catalyst were stirred in a parallel reaction tube for 6 h, at 120 ℃. After the reaction completed, the mixture was filtered at room temperature. The filtrate was determined by GC analysis with the area normalization method. (Conversion rate = 100% × [(S1 × S2)/ S1], S1 is the area of aryl halides before reaction, S2 is the area of aryl halides after the reaction; selectivity = 100% × [S1/(S1 + S2)], S1 is the area of principal product, S2 is the area of all by-products.) The filter cake (Pd NPs/CNFs) was washed with acetone, ethanol, and distilled water three times.
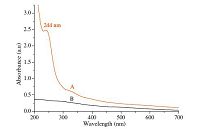
3. Results and discussion 3.1. Characterization of Pd NPs/CNFs catalystThe UV-vis spectra of PdCl2/PAN nanofibers and Pd/PAN nanofibers were shown in Fig. 1. As shown in Fig. 1, the A curve is PdCl2/PAN, and the B curve is Pd/PAN. The absorption peak of A curve in 244 nm is attributed to Pd2+ or PdCl4 2-. The absorption peak at 244 nm disappeared after the PdCl2/PAN was reduced by the hydrazine hydrate, as shown by the B curves. These changes clearly demonstrated that Pd2+ can be reduced into Pd(0) by the hydrazine hydrate solution. At the same time, it also showed that the activity of the catalyst group is divided into Pd(0). The HRTEM and XRD results further proved that PdCl2/PAN was reduced.
|
Download:
|
| Fig. 1.UV-vis spectra of (A) PdCl2/PAN and (B) Pd/PAN. | |
Fig. 2 shows the FT-IR spectra for the PAN fibers, the PdCl2/PAN nanofibers, the Pd/PAN nanofibers, and the Pd NPs/CNFs. For the PAN nanofibers, the IR absorption peak at about 2243 cm-1 was attributed to the stretching vibrations of nitrile groups (-CN), and the peaks at about 2939 cm-1 and 1447 cm-1 were attributed to the stretching vibrations and the bending vibration of methylene (-CH2-), respectively [27, 28]. The peak at about 1736 cm-1 might originate from the vibration of C=O bonds formed in the hydrolyzed PAN nanofibers and the stretching vibration of the C=O bonds in the residual solvent DMF [29]. After the preoxidation and carbonization process, the absorption peaks of the PAN nanofibers all disappeared. For the carbon nanofibers, the IR absorption peaks at 1588 cm-1 and 1292 cm-1 were attributed to the stretching vibrations of C=C bonds, and the stretching vibrations of C-O, respectively [30]. This is because the cyclization reaction, the dehydrogenation reaction, the oxidation reaction happened in the process of preoxidation, and the cyano disconnected in the carbonization process, so the exhaust end discharged NH3, HCN, H2, H2O, etc. [31]. Therefore, FT-IR indicated that the catalyst carrier was the carbon fibers.

|
Download:
|
| Fig. 2.FT-IR spectra of (A) PAN, (B) PdCl2/PAN, (C) Pd/PAN and (D) Pd NPs/CNFs. | |
The structure and morphology of the PAN, PdCl2/PAN, Pd/PAN and Pd NPs/CNFs were characterized by field emission scanning electron microscopy (FE-SEM) and transmission electron microscopy (TEM). The overall morphology of the nanofibers can be seen from the FE-SEM images, and the fibers are not cross-linked and arranged in order. Fig. 3A showed that PAN nanofibers had a large length to diameter ratio and a smooth surface nanostructure. The diameters of PAN nanofibers ranged from 260 nm to 300 nm. After the PdCl2/PAN was reduced by hydrazine hydrate, the diameter of the fibers were changed due to the swelling, as shown in Fig. 3C. After high temperature carbonization, the surface of the Pd NPs/ CNFs was smooth, and it can be observed that a large number of particles were loaded into the carbon fiber matrix, and the particle distribution is uniform. The HRTEM and XRD results confirmed that these particles are palladium nanoparticles.

|
Download:
|
| Fig. 3.FE-SEM images of (A) PAN, (B) PdCl2/PAN, (C) Pd/PAN and (D) Pd NPs/CNFs. | |
The TEM images in Fig. 4 provide a more direct view of the Pd NPs/CNFs, it can be seen that the nanoparticles were loaded and well-dispersed within the carbon fiber matrix. From Fig. 4A, the size distribution of Pd nanoparticles were 11-15 nm. Some of these particles have a small agglomeration phenomenon, which may be formed during the reduction of hydrazine hydrate in an ice bath environment. In addition, HRTEM in Fig. 4B revealed a direct view of the palladium nanoparticles. The clear lattice fringes with an interplanar lattice spacing of ~0.2299 nm corresponded to the (111) atomic planes of face centered cubic Pd. This is consistent with the results of XRD diffraction angle of 40.1°.

|
Download:
|
| Fig. 4.TEM images and particle size distribution histogram of (A) Pd NPs/CNFs; HRTEM images of Pd NPs. | |
XRD patterns of the Pd NPs/CNFs were shown in Fig. 5. The first characteristic diffraction peak at about 24.0° was originated from the CNF carrier. The peaks at 2θ values of 40.1°, 46.6°, 68.1°, 82.1°, and 86.6° are due to the (111), (200), (220), (311), and (222) diffraction peaks of face centered cubic Pd (JCPD-46-1043). So the palladium nanoparticles formed the zerovalent palladium crystal structure, which is in conformity with the UV-vis and HRTEM results. In conclusion, the active center of catalyst is the zerovalent palladium.
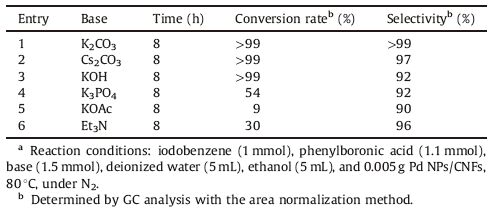
3.2. Catalytic activity of Pd NPs/CNFs in Suzuki-Miyaura carbon coupling reactionThe Pd NPs/CNFs is very stable and high-efficiency in the liquidphase reaction. To verify the practicabilityof the prepared catalyst Pd NPs/CNFs, our study commenced with its application for the Suzuki- Miyaura cross-coupling reaction. The reaction was performed with different alkali salts to investigate their effects on the reaction, and the results are shown in Table 1. By comparison, KOAc, Et3N, and K3PO4 were less effective for the reaction (Table 1, entries 4-6). It is observed that most inorganic bases are more effective (Table 1, entries 1-4) than organic base Et3N (Table 1, entry 6). The results reveal that, in the presence of K2CO3, Cs2CO3, KOH, the conversion rate of iodobenzene is nearly 100% (Table 1, entries 1-3). AsCs2CO3 is radioactive, and when the KOH is present, the Pd NPs/CNFs catalyst exhibited lower selectivity than K2CO3, so K2CO3 was chosen for subsequent investigations.
|
|
Table 1 Suzuki-Miyaura coupling reaction of iodobenzene with phenylboronic acid catalyzed by Pd NPs/CNFs.a |
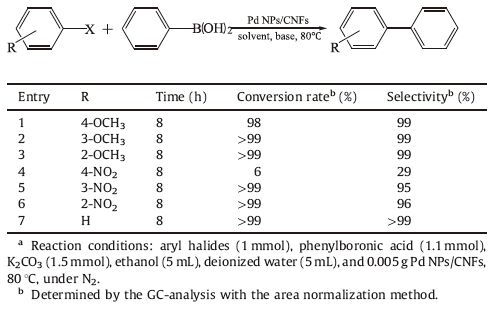
The catalytic activity of Pd NPs/CNFs was tested for the Suzuki- Miyaura reaction of phenylboronic acid and different aryl halides under heterogeneous conditions with K2CO3 as an acid binding agent. The Pd NPs/CNFs showed good catalytic activity to different aryl halides (Table 2, entries 1-3, 5-7). But the conversion rate was low (Table 2, entries 4) when 1-iodo-4-nitrobenzene was used as the substrate, which is due to the poor solubility of 1-iodo-4- nitrobenzene in solvent.
|
|
Table 2 Suzuki-Miyaura coupling reaction of different aryl halides with phenylboronic acid catalyzed by Pd NPs/CNFs.a |
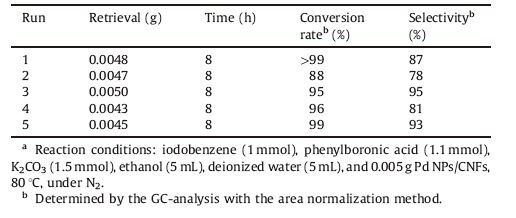
To evaluate the reusability of Pd NPs/CNFs catalyst, the catalyst after completion of the reaction was separated and was washed with acetone, ethanol, and distilled water three times. The dried and the recycled catalyst were reused directly for the next cycle without further treatment. The reaction between phenylboronic acid and iodobenzene was selected to explore the reusability of the catalysts. Generally, the heterogeneous catalysts easily lose their catalytic activity due to the extensive leaching of the active mental species. To our surprise, the Pd NPs/CNFs catalyst can be recycled five times with a negligible drop in activity (Table 3, entries 1-5). Thus, the catalyst is stable during the Suzuki coupling reaction. The good recyclability of Pd NPs/CNFs provides a way to greatly reduce the cost of the catalyst.
|
|
Table 3 Recyclability of Pd NPs/CNFs with iodobenzene catalyzed by Pd NPs/CNFs.a |
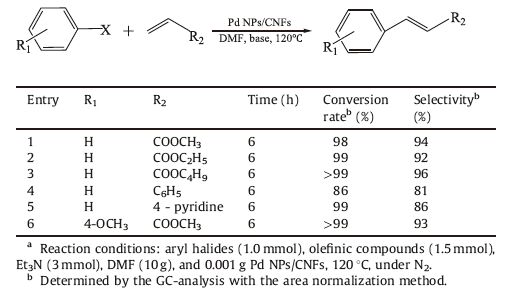
To extend the scope of coupling reaction, we examined the Heck reaction of different aryl iodides with olefinic compounds. As shown in Table 4, the substituents at the phenyl ring of aryl iodides and different olefinic compounds had no significant effect on the reaction consequence. The catalytic activity of Pd NPs/CNFs was high in the Heck reaction. The high catalytic activity of Pd NPs/ CNFs may be ascribed to the large active surface area.
|
|
Table 4 Mizoroki-Heck cross-coupling reaction of aryl halides with olefinic compounds catalyzed by Pd NPs/CNFs.a |
In summary, palladium nanoparticles supported on carbon nanofibers (Pd NPs/CNFs) were successfully prepared by the electrospinning method, hydrazine hydrate solution reduction in an ice bath environment, and high temperature carbonization. The results of FE-SEM and TEM conducted indicate that the arrangement of nanofibers is regular and the palladium nanoparticles are uniformly distributed in the matrix of carbon fibers. The Pd NPs/ CNFs catalyst was successful applied in Suzuki and Heck reaction and exhibited high efficiency, good reusability and stability. It can be used for at least five consecutive runs without a significant loss of its catalytic activity. The good recyclability of Pd NPs/CNFs provides a way to greatly reduce the cost of the catalyst.
AcknowledgmentThe authors gratefully acknowledge the support of the National Natural Science Foundation of China (No. 21266016).
| [1] | P.R. Rao Vadaparthi, C.H. Pavan Kumar, K. Kumar, et al., Synthesis of costunolide derivatives by Pd-catalyzed Heck arylation and evaluation of their cytotoxic activities, Med. Chem. Res. 24(2015) 2871-2878. |
| [2] | S. Das, S. Jana, B. Dutta, S. Koner, Synthesis of symmetrically functionalized oligo (p-phenylenevinylene) by Pd-catalyzed Heck coupling reaction, Res. Chem. (Ⅰ)ntermed. 41(2015) 4825-4832. |
| [3] | P.C. Rodrigues, B.D. Fontes, B.B.M. Torres, et al., Synthesis of a PPV-fluorene derivative:applications in luminescent devices, J. Appl. Polym. Sci. 132(2015) 42579. |
| [4] | (Ⅰ). Favier, D. Madec, E. Teuma, M. Gomez, Palladium nanoparticles applied in organic synthesis as catalytic precursors, Curr. Org. Chem. 15(2011) 3127-3174. |
| [5] | A. Fihri, M. Bouhrara, B. Nekoueishahraki, J.M. Basset, V. Polshettiwar, Nanocatalysts for Suzuki cross-coupling reactions, Chem. Soc. Rev. 40(2011) 5181-5203. |
| [6] | J. Hassan, M. Sévignon, C. Gozzi, E. Schulz, M. Lemaire, Aryl-aryl bond formation one century after the discovery of the Ullmann reaction, Chem. Rev. 102(2002) 1359-1470. |
| [7] | X.M. Chen, G.H. Wu, J.M. Chen, et al., Synthesis of "Clean" and well-dispersive Pd nanoparticles with excellent electrocatalytic property on graphene oxide, J. Am. Chem. Soc. 133(2011) 3693-3695. |
| [8] | G.M. Scheuermann, L. Rumi, P. Steurer, W. Bannwarth, R. Mülhaupt, Palladium nanoparticles on graphite oxide and its functionalized graphene derivatives as highly active catalysts for the Suzuki-Miyaura coupling reaction, J. Am. Chem. Soc. 131(2009) 8262-8270. |
| [9] | M.X. Chen, Z. Zhang, L.Z. Li, et al., Fast synthesis of Ag-Pd@reduced graphene oxide bimetallic nanoparticles and their applications as carbon-carbon coupling catalysts, RSC Adv. 4(2014) 30914-30922. |
| [10] | M. Amini, A. Tarassoli, S. Yousefi, et al., Suzuki-Miyaura cross-coupling reactions in water using in situ generated palladium(Ⅱ)-phosphazane complexes, Chin. Chem. Lett. 25(2014) 166-168. |
| [11] | M. Bakherad, A. Keivanloo, B. Bahramian, S. Jajarmi, Suzuki, Heck, and copper-free Sonogashira reactions catalyzed by 4-amino-5-methyl-3-thio-1, 2, 4-triazolefunctionalized polystyrene resin-supported Pd(Ⅱ) under aerobic conditions in water, J. Organomet. Chem. 724(2013) 206-212. |
| [12] | Y.H. Qin, Y. Jiang, H.H. Yang, et al., Synthesis of highly dispersed and active palladium/carbon nanofiber catalyst for formic acid electrooxidation, J. Power Sources 196(2011) 4609-4612. |
| [13] | Y.S. Feng, X.Y. Lin, J. Hao, H.J. Xu, Pd-Co bimetallic nanoparticles supported on graphene as a highly active catalyst for Suzuki-Miyaura and Sonogashira crosscoupling reactions, Tetrahedron 70(2014) 5249-5253. |
| [14] | S. Keesara, S. Parvathaneni, G. Dussa, M.R. Mandapati, Polystyrene supported thiopseudourea Pd (Ⅱ) complex:applications for Sonogashira, Suzuki-Miyaura, Heck, Hiyama and Larock heteroannulation reactions, J. Organomet. Chem. 765(2014) 31-38. |
| [15] | L.J. Shao, C.Z. Qi, Supported palladium nanoparticles on preoxidated polyacrylonitrile fiber mat for coupling reactions, Fibers Polym. 15(2014) 2233-2237. |
| [16] | M. Ghiaci, D. Valikhani, Z. Sadeghi, Synthesis and characterization of silicasupported Pd nanoparticles and its application in the Heck reaction, Chin. Chem. Lett. 23(2012) 887-890. |
| [17] | H. Yang, D. Shi, S.F. Ji, D.N. Zhang, X.F. Liu, Nanosized Pd assembled on superparamagnetic core-shell microspheres:synthesis, characterization and recyclable catalytic properties for the Heck reaction, Chin. Chem. Lett. 25(2014) 1265-1270. |
| [18] | S. Jadhava, A. Kumbharb, R. Salunkhe, Palladium supported on silica-chitosan hybrid material (Pd-CS@SiO2) for Suzuki-Miyaura and Mizoroki-Heck crosscoupling reactions, Appl. Organomet. Chem. 29(2015) 339-345. |
| [19] | C.C. Huang, C. Li, G.Q. Shi, Graphene based catalysts, Energy Environ. Sci. 5(2012) 8848-8868. |
| [20] | T. Van Haasterecht, C.C.(Ⅰ). Ludding, K.P. De Jong, J.H. Bitter, Stability and activity of carbon nanofiber-supported catalysts in the aqueous phase reforming of ethylene glycol, J. Energy Chem. 22(2013) 257-269. |
| [21] | L.J. Shao, C.Z. Qi, Preoxidated polyacrylonitrile fiber mats supported copper catalyst for Mizoroki-Heck cross-coupling reactions, Appl. Catal. A 468(2013) 26-31. |
| [22] | J. Kang, R.R. Han, J. Wang, et al., (Ⅰ)n situ synthesis of nickel carbide-promoted nickel/carbon nanofibers nanocomposite catalysts for catalytic applications, Chem. Eng. J. 275(2015) 36-44. |
| [23] | Z.Y. Wu, X.X. Xu, B.C. Hu, et al., (Ⅰ)ron carbide nanoparticles encapsulated in mesoporous Fe-N-Doped carbon nanofibers for efficient electrocatalysis, Angew. Chem. (Ⅰ)nt. Ed. 54(2015) 8179-8183. |
| [24] | J. Zhu, J.H. Zhou, T.J. Zhao, et al., Carbon nanofiber-supported palladium nanoparticles as potential recyclable catalysts for the Heck reaction, Appl. Catal. A 352(2009) 243-250. |
| [25] | X.W. Peng, W. Ye, Y.C. Ding, et al., Facile synthesis, characterization and application of highly active palladium nano-network structures supported on electrospun carbon nanofibers, RSC Adv. 4(2014) 42732-42736. |
| [26] | L.P. Guo, J. Bai, C.P. Li, et al., Fabrication of palladium nanoparticles-loaded carbon nanofibers catalyst for the Heck reaction, N. J. Chem. 37(2013) 4037-4044. |
| [27] | W.X. Zhang, J. Liu, G. Wu, Evolution of structure and properties of PAN precursors during their conversion to carbon fibers, Carbon 41(2003) 2805-2812. |
| [28] | C.Y. Su, J. Liu, C.L. Shao, Y.C. Liu, Controlled synthesis of PAN/Ag2S composites nanofibers via electrospinning-assisted hydro (solvo) thermal method, J. Non-Cryst. Solids 357(2011) 1488-1493. |
| [29] | Y. Wang, J. Liu, J.Y. Liang, Thermo-chemical reactions of modified PAN fibers during heat-treatment process, Adv. Mater. Res. 11-12(2006) 73-76. |
| [30] | L.P. Guo, J. Bai, J.Z. Wang, et al., Fabricating series of controllable-porosity carbon nanofibers-based palladium nanoparticles catalyst with enhanced performances and reusability, J. Mol. Catal. 400(2015) 95-103. |
| [31] | S.J. Zhang, S.X. Chen, Q.K. Zhang, P.Y. Li, C.E. Yuan, Preparation and characterization of an ion exchanger based on semi-carbonized polyacrylonitrile fiber, React. Funct. Polym. 68(2008) 891-898. |
 2016, Vol.27
2016, Vol.27