Aromatic polyimides (PIs) are one of the most important classes of high-performance polymers generally exhibiting outstanding mechanical properties, thermo-oxidative stability, electrical properties, chemical resistance, and have manifold applications as composite matrices, adhesives, coatings, fibers, membranes, etc. [1]. However, most PIs have two main flaws: Bad optical performance and limited process-ability, because of their strong inter- or intramolecular charge transfer complexes and highly conjugated aromatic structures. Additionally, the limiting options of dianhydrides such as expensive prices and complicated synthetic routes also severely hinder the development of polyimides.
Symmetry is a strong and powerful framework to perceive and predict the physicalworld. Triptycene is the simplestmember of the iptycenes assembled with a specific three-dimensional scaffold in which arene rings are held together with a bicycle [2.2.2] octane central unit [2]. Upon the designed organic molecules that filled space in a hexagonal tiling and a propeller-like triptycene base adhered to crystalline surfaces and alkyl tails extendedaway fromit, Seiki et al. could make well-ordered multilayer films up to centimeter length scales [3]. Triptycene has a rigid and shapepersistent D3h symmetry with high degree of internalmolecular free volume. So the triptycene moiety, as a special component, was widely inserted into polymer chains in order to get polymers with desiredproperties [4], suchas lowdielectric constant, materialswith improvedmechanical properties [5], highBET surface areas [6], good solubilities [7] and high-performance gas separation [8], etc.
Herein, a novel triptycene-based dianhydride was conveniently synthesized via a solvothermal method. Similarly, from the perspective of Stephen Z. D. Cheng [9, 10], triptycene as a shapepersistent building block with high internal free volume and D3h symmetry will largely enrich the chemistry of dianhydrides. The research of triptycene-based dianhydrides and polyimides has been ongoing for an extensive time in our group [11, 12]. In this work, three all aromatic polyimides, PIa-PIc, were prepared using triptycene-2,3,6,7-tetracarboxylic dianhydride with three different benzidines through one step polymerization. As expected, we have incorporated the triptycene unit into the structure of dianhydride to obtain polyimides with thermostability, good solubilities, excellent transparency, and high BET surface areas.
2. Experimental 2.1. MaterialsThe components 1,2-dichloroethane (DCE, Sinopharm Chemical Reagent Co., Ltd.), isoquinoline (J&K Scientific Ltd.) and 3,3',5,5'- tetramethylbenzidine (TMB, TCI), o-tolidine (DMB, TCI), 2,2'-bis(trifluoromethyl)benzidine (TFMB, TCI) were used as received. However, m-cresol (J&K Scientific Ltd.) was distilled under reduced pressure using P2O5 as desiccant. Isopentyl nitrite and 2-aminobenzoic acid were synthesized as the Organic Chemistry Experiment of Wang Qinglian published by Higher Education Press of China in 1994. Both 2,3,6,7-tetramethyltriptycene (2) and triptycene-2,3,6,7-tetracarboxylic acid (3) were prepared as described in the literature [13]. All other solvents and reagents were obtained from various commercial sources and used as the AR grade level as received.
2.2. General preparation procedure and characterizations for target compounds2,3,6,7-Tetramethylanthracene (1): A three-neck 500 mL round-bottom flask containing dichloromethane (104 mL, 1.62 mol) and o-xylene (98 mL, 0.81 mol) was stirred under the salt-ice bath. Aluminium trichloride (80 g) was added portion wise to the flask between -5 and 5 ℃. The reaction mixture was then stirred at r.t. for 0.5 h. Then, the mixture was slowly heated to 75 ℃ and stirred for another 4 h. Upon completion, the reaction mixture was poured into water (400 mL) and H2SO4 (3 mol/L, 100 mL), the precipitated product was filtered and crystallized from xylene. The off-white product was filtered and dried at 80 ℃ in vacuum. The yield of compound 1 was 19 g (20%), mp = 299 ℃ (lit. [14] 299 ℃); 1H NMR (400 MHz, CDCl3): δ 2.43 (s, 12H), 7.68 (s, 4H), 8.13 (s, 2H).
Triptycene-2,3,6,7-tetracarboxylic dianhydride (4): A mixture of AcOH (50 mL), Ac2O (10 mL) and triptycene-2,3,6,7-tetracarboxylic acid (3,1 g), was sealed in a 100 mL Teflon-lined stainless autoclave and heated to 160 ℃ for 4 h. After it was slowly cooled to r.t. and subjected to filtration, yellowish crystals of monomer 4 were recovered at 0.55 = g (60%) after drying under vacuum in 120 ℃, mp >300 ℃. 1H NMR (400 MHz, DMSO-d6): δ 6.36 (s, 2H), 7.11-7.14 (m, 2H), 7.57-7.59 (m, 2H), 8.17 (s, 4H). 13C NMR (100 MHz, DMSO-d6): δ 162.9, 152.3,141.8, 139.8, 126.5, 124.9, 120.8, 52.6. FT-IR (KBr, cm-1): 1841 (asym C-O, str), 1780 (sym C=O, str), 1271 (C-O, str), 732 (C-H, str).
Crystal data for monomer 4: C24H10O6, yellowish, M+ = 394.32, monoclinic, space group: C 2/c. a = 15.248(2), b = 8.0812(6), c = 16.473(2) Å ; V = 1706.8(5)Å 3; α = 90.00, β = 122.77, γ = 90.00, Z = 4, T = 293 K, F000 = 808.0, R1 = 0.0476, wR2 = 0.1168.
2.3. Typical preparation procedure and characterizations for triptycene-based polyimide, PIa, PIb, PIcTo a dry 25 mL two-neck, round-bottomed flask equipped with a Dean-Stark trap with a nitrogen inlet were added the diamine (0.5 mmol) and an equimolar amount of dianhydride monomer (0.1972 g, 0.5 mmol), the mixture were added freshly distilled m-cresol (3 mL), toluene (1 mL) and isoquinoline (3 drops). The reaction mixture was stirred at r.t. for 1 h, and the temperature was raised gradually to 200 ℃ and held for 4 h under a nitrogen atmosphere. After cooling to r.t., fibrous polyimide was obtained by the addition of the polymer solution to methanol. The resulting solid was filtered, the polymer was dissolved in DMAc and washed sequentially with toluene, chloroform, and methanol. Finally, the polymers were purified for 24 h in a Soxhlet apparatus with methanol and dried at 150 ℃ for 12 h.
PIa: Following the above general procedure, PIa was prepared from TFMB and monomer 4. White fibrous powder, yield: 0.34 g, 97%; 1H NMR (500 MHz, DMSO-d6): δ 8.0-8.2 (br m, 4H), 7.8-8.0 (br m, 2H), 7.3-7.8 (br m, 4H), 7.0-7.3 (br m, 2H), 6.2-6.4 (br m, 2H). FT-IR (Film, cm-1) υ 1778 (asym C=O, str), 1724 (sym C=O, str), 1368 (C-N, str), 742 (imide ring deformation). GPC (THF): Mw = 6.9 ± 104 g mol-1, Mn = 4.8 ± 104 g mol-1 relative to polystyrene, Mw/Mn = 1.4. BET surface area = 457 m2 g-1, TGA analysis: (nitrogen), the 5 wt% thermal degradation commences at 463 ℃.
PIb: Following the above general procedure, PIb was prepared from DMB and monomer 4. White fibrous powder, yield: 0.27 g, 94%; 1H NMR (500 MHz, DMSO-d6): δ 7.8-8.3 (br m, 6H), 7.45-7.8 (br m, 2H), 6.9-7.45 (br m, 6H), 6.1-6.5 (br m, 2H), 1.8-2.3 (br m, 6H). FT-IR (Film, cm-1) υ 1774 (asym C=O, str), 1720 (sym C=O, str), 1364 (C-N, str), 750 (imide ring deformation). BET surface area = 481 m2 g-1, TGA analysis: (nitrogen), the 5 wt% thermal degradation commences at 443 ℃.
PIc: Following the above general procedure, PIc was prepared from TMB and monomer 4. White fibrous powder, yield: 0.29 g, 96%; 1H NMR (500 MHz, DMSO-d6): δ 8.09-8.43 (br m, 4H), 7.43-7.86 (br m, 6H), 7.02-7.30 (br m, 2H), 6.21-6.55(br m, 2H), 1.77-2.33(br m, 12H). FT-IR (Film, cm-1) υ 1774 (asym C=O, str), 1720 (sym C=O, str), 1368 (C-N, str), 743 (imide ring deformation). BET surface area = 623 m2 g-1,TGA analysis: (nitrogen), the 5 wt% thermal degradation commences at 520 ℃.
3. Results and discussionScheme 1 shows that compound 1 was obtained in 20% yield through a one-pot reaction starting from a relay domino tandem FC reaction of o-xylene and dichloromethane, and subsequent aromatization reaction. It is fortunate that we could easily get a large quantity of compound 1, since it is not available commercially. The 1H NMR analysis of compound 1 shows that no other isomers are generated in the reaction. Triptycene-based dianhydride 4 was readily synthesized in three steps from the anthranilic acid and compound 1. Compound 2 was prepared in 79% yield under very mild conditions with high efficiency by the Diels-Alder addition between compound 1 and benzyne, which was formed in situ from 2-aminobenzoic acid. The product was confirmed by 1H and 13C NMR analysis and no byproducts were detected. Then, compound 2 was oxidized to form tetracarboxylic acid in 92% yield by potassium permanganate.

|
Download:
|
| Scheme 1. Synthesis of triptycene-based dianhydride monomer (4) and the ORTEP of monomer 4. | |
The dianhydride can be obtained through dehydration of the corresponding tetracarboxylic acid by different approaches, such as sublimation, catalysis, and in organic solvents, etc. However, the thermal dehydration of tetracarboxylic acids is usually performed at high temperature, which inevitably makes the raw materials partially carbonized. And the preparation of catalyst as dehydrant is complicatedand expensive by the industrial method[15]. Therefore, most of the aromatic carboxylic dianhydrides are prepared fromthe corresponding tetracarboxylic acids using organic solvents as dehydration and recrystallization reagents, with acetic anhydride and acetic acid often used. Unfortunately, triptycene-based dianhydride shows very poor solubility in acetic anhydride or acetic acid under normal conditions due to the high rigidity and symmetry of triptycene. On the other hand, the triptycene-based dianhydride was obtained in low yield by sublimation.
Recently, we demonstrated that triptycene-based dianhydride 4 can be directly obtained through dehydration of the corresponding tetracarboxylic acids by a solvothermal method. The experiments were performed in a mixture of acetic anhydride and acetic acid under conditions of high temperature and high pressure, as triptycene-based dianhydrides is supposed to be soluble in the medium [16]. The solvothermal time and temperature were optimized to make compound 3 dehydrated and make monomer 4 dissolve thoroughly, and the optimum ratio of acetic anhydride and acetic acid is 1:5, yield: 60%. The X-ray diffraction of monomer 4 demonstrates that the triptycene-based dianhydrides was obtained as single crystal in a high purity. A solvothermal process is defined as a chemical reaction in a closed system in the presence of a solvent at a temperature higher than the boiling point of the solvent and is mainly used for preparing inorganic micro- or nanoparticles with different morphologies, nanocrystals [17], metal-organic framework [18], etc. To our knowledge, this is the first report on the application of a solvothermal method in the synthesis of aromatic dianhydrides.
The dihedral angle of monomer 4 is 110.625°, it suggests that polyimides synthesized by monomer 4 are inclined to form cyclic oligomer which has a serious impact on the performances of polyimide [19]. In general, the preparation of polyimide via poly(amic acid) precursor has some inherent problems, such as emission of volatile byproducts and storage instability of the poly(amic acid) solution. In order to avoid these drawbacks, we conducted polymerization of monomer 4 with three benzidines (TFMB, DMB, TMB) by a one-step method, as show in Scheme 2. The 1H NMR spectrum of PIa is shown in Fig. 1a, all the signals of the aromatic protons in PIa appear in the region of δ 6.0-8.5 ppm. The signals appearing in the range 6.0-6.5 ppm were assigned to the bridgehead protons of the triptycene units. Assignments of each proton are in good agreement with the structure of their repeating units.
|
Download:
|
| Scheme 2.Synthesis of triptycene-based polyimides PIa-PIc. | |

|
Download:
|
| Fig. 1.(a) 1H NMR spectrum of PIa and (b) WXAD patterns of PIa-PIc. | |
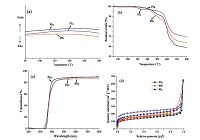
The crystallinity of the polyimide powders were evaluated by wide-angle X-ray diffraction (WAXD), as shown in Fig. 1b. All the aromatic polyimides appeared amorphous, presumably due to the existence of the shape-persistent triptycene unitwith large internal free volume. The PIa, which is soluble in THF, was characterized by GPC, Mw = 6.9 ± 104 g mol-1, Mn = 4.8 ± 104 g mol-1,The data listing the thermal properties and solubility of PIa-PIc are found in Table 1,and their correspondingDSC andTGAcurves are shownin Fig. 2a and b. In the range of 50-300 ℃, all the polymers had no detectable glass transition. Weight losses were observed in the temperature range of 443-520 ℃. Moreover, PIa, PIb, and PIc displayed high char yields of 59%-70% in nitrogen at 800 ℃. Fig. 2c shows the UV-vis spectra of the PIa-PIc; membranes in -20 mm thickness cast from solvent is highly transparent (~85% at 450 nm) even though the chain of PIa-PIc is fully aromatic and rigid. Thismay be attributed to the contorted and rigid structure of the polymer chains. In addition, the third benzene ring may have the effect of a baffle, which hindered the formation of charge transfer complexes.
|
|
Table 1 Properties of triptycene-based polyimide PIa-PIc. |
|
Download:
|
| Fig. 2.(a) DSC curves, (b) TGA curves, (c) UV-vis spectrum and (d) Nitrogen sorption isotherms at 77 K of PIa-Pic. | |
Fig. 2d shows the nitrogen sorption isotherms of PIa-PIc at 77 K. The amount of BET surface areas of PIa-PIc may be associated with the internal free volume of triptycene moieties and consequently the disrupted chain packing [20]. So, the introduction of various substituted groups probably affected the fractional free volume of the PIa-PIc, which led to BET surface area of PIc (623 m2 g-1) showing a higher value than the BET surface areas of PIa (457 m2 g-1) and PIb (481 m2 g-1). The BET surface areas of the PIa-PIc suggest that chain rigidity plays a crucial role in the aggregation morphology. PIa has a much higher BET surface area of 457 m2 g-1 compared to 6FDA-DATRI which contains imide linkages to a flexible 2,6-diaminotriptycene (BET surface area, 68 m2 g-1) [7] and the polyimide synthesized by 6FDA and 2,6-diamino-13,14, 15, 16-tetrachlorotriptycene diamine (BET surface area, 370 m2 g-1) [21]. It is apparent that when triptycene is fixed in dianhydrides, it will greatly enhance the chain rigidity. Similar evidence has been shown in KAUST-PIs (BET surface area, 420-840 m2 g-1) [8], however compared to the preparation of monomer 4, the synthesis of the dianhydride is much more complicated. The pore size distribution of PIa-PIc was calculated by NLDFT method, and the results were shown in Fig. S8 in Supporting information.
4. ConclusionIn conclusion, a new strategy for the preparation of triptycene dianhydride has been developed, and its structure determined by 1H NMR, 13C NMR, FT-IR spectra and X-ray single crystal diffraction. Then, three all-aromatic polyimides based on the triptycene dianhydride were successfully synthesized. The triptycene unit as a shape-persistent building block with a high internal free volume and D3h symmetry was incorporated into the polyimide, which showed high performance characteristics, such as thermostability, good solubilities, excellent transparency, and high BET surface areas. This work provides a promising new approach for the design and synthesis of new polyimides exhibiting high performance.
It is serendipity for us to find such a virgin field that a library of new dianhydrides incorporating the triptycene as an element with high internal free volume and D3h symmetry can be synthesized through 2,3,6,7-tetramethylanthracene with different benzyne equivalents with further oxidation and dehydration using solvothermal method. Utilizing this principle, these new dianhydrides can be polymerized with equimolar diamines to synthesize polyimides. New building blocks will enable the design of materials with previously unknown properties, hence, other triptycene-based dianhydride derivatives are intensely under study in our group.
AcknowledgmentWethank the National Natural Science Foundation of China (No. 514730=) for financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2015.12.018.
| [1] | D.J. Liaw, K.L. Wang, Y.C. Huang, et al., Advanced polyimide materials:syntheses, physical properties and applications, Prog. Polym. Sci. 37(2012) 907-974. |
| [2] | P.D. Bartlett, M.J. Ryan, S.G. Cohen, Triptycene1(9,10-o-benzenoanthracene), J. Am. Chem. Soc. 64(1942) 2649-2653. |
| [3] | N. Seiki, Y. Shoji, T. Kajitani, et al., Rational synthesis of organic thin films with exceptional long-range structural integrity, Science 348(2015) 1122-1126. |
| [4] | T.M. Swager, (Ⅰ)ptycenes in the design of high performance polymers, Acc. Chem. Res. 41(2008) 1181-1189. |
| [5] | N.T. Tsui, A.J. Paraskos, L. Torun, T.M. Swager, E.L. Thomas, Minimization of internal molecular free volume:amechanismfor the simultaneous enhancement ofpolymer stiffness, strength, and ductility, Macromolecules 39(2006) 3350-3358. |
| [6] | M. Carta, M. Croad, R. Malpass-Evans, et al., Triptycene induced enhancement of membrane gas selectivity for microporous Tröger's base polymers, Adv. Mater. 26(2014) 3526-3531. |
| [7] | S.A. Sydlik, Z.H. Chen, T.M. Swager, Triptycene polyimides:soluble polymers with high thermal stability and low refractive indices, Macromolecules 44(2011) 976-980. |
| [8] | B.S. Ghanem, R. Swaidan, E. Litwiller, (Ⅰ). Pinnau, Ultra-microporous triptycenebased polyimide membranes for high-performance gas separation, Adv. Mater. 26(2014) 3688-3692. |
| [9] | M.J. Huang, C.H. Hsu, J. Wang, et al., Selective assemblies of giant tetrahedra via precisely controlled positional interactions, Science 348(2015) 424-428. |
| [10] | W.B. Zhang, X.F. Yu, C.L. Wang, et al., Molecular nanoparticles are unique elements for macromolecular science:From "nanoatoms" to giant molecules, Macromolecules 47(2014) 1221-1239. |
| [11] | L. Cheng, X.Q. Xiong, Z. Xu, B. Jing, J.X. Wang. Procedures for preparation of triptycene 2,3,6,7-tetracarboxylic dianhydride. Patent CN101481378B. |
| [12] | Z. Xu, X.Q. Xiong, L. Cheng, Novel hyperbranched polyimides from 2,6,12-triaminotriptycene, Chin. Chem. Lett. 19(2008) 1127-1130. |
| [13] | M. Rybáčková, M. Bělohradský, P. Holý, et al., Synthesis of highly symmetrical triptycene tetra-and hexacarboxylates, Synthesis 10(2007) 1554-1558. |
| [14] | E. De Barry Barnett, N.F. Goodway, J.W. Watson, Beiträge zur Kenntnis der Anthracen-Derivate (X. Mitteil.), Ber. Dtsch. Chem. Ges. 66(1933) 1876-1891. |
| [15] | A. Sakakura, T. Ohkubo, R. Yamashita, M. Akakura, K. (Ⅰ)shihara, Brønsted baseassisted boronic acid catalysis for the dehydrative intramolecular condensation of dicarboxylic acids, Org. Lett. 13(2011) 892-895. |
| [16] | G. Demazeau, A. Largeteau, Hydrothermal/solvothermal crystal growth:an old but adaptable process, Z. Anorg. Allg. Chem. 641(2015) 159-163. |
| [17] | K. Namratha, K. Byrappa, Novel solution routes of synthesis of metal oxide and hybrid metal oxide nanocrystals, Prog. Cryst. Growth Charact. Mater. 58(2012) 14-42. |
| [18] | O.K. Farha, J.T. Hupp, Rational design, synthesis, purification, and activation of metal-organic framework materials, Acc. Chem. Res. 43(2010) 1166-1175. |
| [19] | X.Z. Fang, Z.H. Yang, S.B. Zhang, L.X. Gao, M.X. Ding, Polyimides derived from mellophanic dianhydride, Macromolecules 35(2002) 8708-8717. |
| [20] | S.J. Luo, Q. Liu, B.H. Zhang, et al., Pentiptycene-based polyimides with hierarchically controlled molecular cavity architecture for efficient membrane gas separation, J. Membr. Sci. 480(2015) 20-30. |
| [21] | X.J. Zheng, Y. Shao, Y.H. Xiao, S.H. Du, L. Cheng, Synthesis and properties of polyimides derived from triptycene with D3h symmetry, Acta Polym. Sin. 11(2014) 1501-1507. |
 2016, Vol.27
2016, Vol.27