b. National Engineering Research Center for Dyeing & Finishing of Textiles, Shanghai 201620, China
Silver sulfide (Ag2S) is a direct, narrow-band gap semiconductor with good chemical stability, excellent photoelectric and thermoelectric properties, and has a band gap of Eg ≈ 1 eV at room temperature [1, 2, 3, 4, 5, 6, 7]. Ag2S in different morphologies, including monodispersed particles [8], rod-shaped [9, 10], wire-shaped [1, 11, 12], rub-shaped [13] and hollow particles [14], have unique physical and chemical properties and wide potential applications and have attracted much attention to their synthesis.
In some reports, with Ag+-TU complexes as precursors, Ag2S with different morphologies were synthesized using different methods. Lu et al. [15] prepared quasi-network Ag2S microstructure from a AgNO3-TU complex using the hydrothermal method; Dong et al. [16] prepared faceted and cubic Ag2S nanocrystals in aqueous solutions from AgNO3, TU and CTAB using the hydrothermal method; Sahraoui et al. [17] prepared Ag2S thin films from AgCl and TU using the spray pyrolysis method. However, there is no report on the preparation of hollow carambola-shaped Ag2S microspheres with large specific surface area owing to their special morphology and hollow structure [18].
Microwave technology is a new synthetic technology for materials, which has some advantages, such as uniform heating, no-hysteresis effect, energy-efficiency, safety and greenness [19]. The hollow carambola-shaped silver sulfide microspheres were prepared with silver nitrate, thiourea, sodium chloride and diethanolamine as raw materials using a microwave-assisted method, at low temperatures of below 100 ℃ in an open system.
2. ExperimentalPreparation of hollow carambola-shaped Ag2S microspheres on cotton fabric: In a typical synthetic process, a piece of cotton fabric was put in a three-necked bottle. After that, 50 mL of 0.04 mol L-1 TU, 25 mL of 0.04 mol L-1 AgNO3 aqueous solutions and 25 mL of 0.04 mol L-1 NaCl aqueous solutions were added into the bottle separately under magnetic stirring. The mixture was maintained at 4 ℃ for 2 h without stirring to form a white precipitate on fabric. After 5 mL of diethanolamine was added under magnetic stirring, the system was heated under microwave irradiation at 80 ℃ and normal atmosphere for 15 min in a microwave oven (Beijing XiangHu Science and Technology Development Reagent Co., Ltd., equipped with a magnetic stirring system and a water-cooled condenser outside the oven). After the system was cooled to room temperature, finished cotton fabric was taken out and washed several times with deionized water and dried by vacuum freeze- drying for 12 h at -50 ℃.
3. Results and discussionAg2S was synthesized in two steps from AgNO3. The precursor was first prepared by mixing AgNO3 aqueous solutions and TU aqueous solutions with a molar ratio of Ag:TU = 1:2 [20]. After the addition of NaCl, the mixture was kept at 4 ℃ for 2 h to yield a white precipitate [Ag(TU)]Cl. With the help of microwave heating, [Ag(TU)]Cl was hydrolyzed and S2- anions were released. The S2- anion reacted with Ag+ in the precursor to form Ag2S. The related reactions are shown in Scheme 1.

|
Download:
|
| Scheme 1.Synthesis of Ag2S from AgNO3. | |
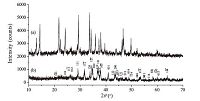
The white precipitate was first proposed as [Ag(TU)2]Cl. However, the results of elemental analysis was consistent with the theoretical weight percent of [Ag(TU)]Cl (see data in Supporting information). The XRD patterns of [Ag(TU)]Cl and carambola-shaped Ag2S microsphere are shown in Fig. 1. All peaks in Fig. 1b can be indexed to monoclinic α-Ag2S (JCPDS No.14-0072) with lattice constants of a = 0.4229 nm, b = 0.6931 nm and c = 0.7862 nm. The absence of impurity phases, such as [Ag(TU)]Cl phase (Fig. 1a), in the final products indicated the high purity of Ag2S.
|
Download:
|
| Fig. 1.XRD patterns of [Ag(TU)]Cl (a) and Ag2S (b). | |
The SEM images of [Ag(TU)]Cl and carambola-shaped Ag2S microspheres are shown in Fig. 2. It can be seen that [Ag(TU)]Cl microspheres had good monodispersity with diameters of about 2.4 μm in width and ~2.0 μm in length. The hexapetalous carambola-shaped Ag2S microspheres consist of longitudinal ridges and the ridges are around ~720 nm in width and ~900 nm in thickness (Fig. 2b). From Fig. 2c, it can be seen that there were massive Ag2S microspheres with uniform sizes on cotton fiber. Fig. 2d shows the detailed morphology of carambolashaped Ag2S microspheres with a uniform architecture of ~1.0 μm in width and ~1.5 μm in length. The carambola consists of six longitudinal ridges and the ridges are around ~475 nm in width and ~375 nm in thickness. It should be pointed out that the morphology of carambola-shaped Ag2S remained basically unchanged after microwave-heating at the presence of DEA. Interestingly, the carambola-shaped Ag2S is a hollow structure. Compared with [Ag(TU)]Cl, no significant changes were observed, except the reduced size, coarse surface and small holes on the microsphere (the marking ring in Fig. 2d). This confirmed the successful realization of morphology transfer from one material to another.

|
Download:
|
| Fig. 2.SEM images of [Ag(TU)]Cl and Ag2S microspheres with different magnifications: (a and b) [Ag(TU)]Cl; (c and d) Ag2S microspheres. | |
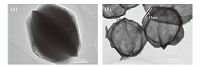
The TEM image of [Ag(TU)]Cl and Ag2S are shown in Fig. 3. It could be seen that carambola-shaped [Ag(TU)]Cl microsphere is solid. The Ag2S microsphere contains hollow carambola-shaped structures, in which the light color part showed hollow structure and the deep color showed layer structure with a thickness of about = nm. The diameters of Ag2S microspheres are about 1.5 μm.
|
Download:
|
| Fig. 3.TEM images of [Ag(TU)]Cl and Ag2S microspheres: (a) [Ag(TU)]Cl; (b) Ag2S microspheres. | |
Based on the above results, the formation mechanism of hollow carambola-shaped Ag2S microspheres can be envisioned as a twostage process (Fig. 4). The first stage is the formation of [Ag(TU)]Cl and nucleation of amorphous precursors. As an electroneutral ligand, thiourea forms complex [Ag(TU)]Cl with Ag+ through Ag-S coordination bonding, which promoted the oriented growth of the hierarchical structures of solid carambola-shaped [Ag(TU)]Cl microspheres assembled from [Ag(TU)]Cl molecules. The bridging mode of TU for polymeric chains [20] and the hydrogen bond N- H…Cl [21] further kept the polymeric chains together. All the informed factors influenced the nucleation to form solid carambola- shaped [Ag(TU)]Cl microspheres.

|
Download:
|
| Fig. 4.Schematic illustration of the formation of carambola-shaped silver sulfide hierarchical structure. | |
The second stage is a microwave-assisted localized Ostwald ripening, involving the chemically induced self-transformation associated with preferential dissolution of the particle interior. Thiourea in [Ag(TU)]Cl was first decomposed to release S2- to generate Ag2S nucleuses preferentially at the surface of carambolashaped [Ag(TU)]Cl microspheres under microwave heating. The interactions between S2- and [Ag(TU)]Cl surface are optimal for localized Ostwald ripening, using [Ag(TU)]Cl microspheres as selfsacrificial templates and both the silver and sulfur sources for the outer Ag2S [22]. As a result, the smooth solid complex carambolashaped [Ag(TU)]Cl microspheres gradually converted into carambola- shaped Ag2S microspheres with hollow structures. During the processing, diethanolamine acted both as an activator and a controller of the decomposition of thiourea in [Ag(TU)]Cl microspheres. The alkalescence of diethanolamine further prevented the hollow carambola-shaped structure from collapsing. Due to the inner penetration and faster heating, microwave-assisted heating facilitated the quick formation, nucleation and growth of Ag2S occur in situ and grew uniformly at the surface of microspheres to form the complete hollow carambola-shaped structure.
4. ConclusionThe hollow carambola-shaped Ag2S microspheres were successfully prepared with silver nitrate, thiourea, sodium chloride and diethanolamine as raw materials on cotton fabric using a microwave-assisted method, at low temperatures of below 100 ℃ in an open system. The results of XRD indicated that the Ag2S prepared is monoclinic Ag2S with high purity. SEM and HRTEM indicated that the structure of Ag2S microspheres prepared is hollow carambola-shaped and the diameter was about 1.5 μm. This simple method is efficient and easy to control the hollow carambola-shaped structure, and to successfully combine textile materials, which give us a good grounding in researching the applications of Ag2S in the textile materials field.
AcknowledgmentsThis work was supported by the Fundamental Research Funds for the Central Universities (No. 2232013A3-05) and the National Science and Technology Ministry (No. ID 2012BAK30B03).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2015.12.009.
| [1] | X.N. Li, X.C. Yang, S.S. Han, et al., Synthesis and characterization of high density and high aspect ratio Ag2S nanoparticle nanowires from a paired cell method, Chin. Sci. Bull. 56(2011) 1828-1831. |
| [2] | W.P. Lim, Z.H. Zhang, H.Y. Low, W.S. Chin, Preparation of Ag2S nanocrystals of predictable shape and size, Angew. Chem. (Ⅰ)nt. Ed. 116(2004) 5803-5807. |
| [3] | S.C. Han, L.F. Hu, N. Gao, A.A. Al-Ghamdi, X.S. Fang, Efficient self-assembly synthesis of uniform CdS spherical nanoparticles-Au nanoparticles hybrids with enhanced photoactivity, Adv. Funct. Mater. 24(2014) 3725-3733. |
| [4] | W.W. Cai, H. Yang, X.Z. Guo, A facile one-step route to synthesize titania hollow microspheres with incontinuous multicavities, Chin. Chem. Lett. 25(2014) 441-446. |
| [5] | M. Chen, L.F. Hu, J.X. Xu, et al., ZnO hollow-sphere nanofilm-based high-performance and low-cost photodetector, Small 7(2011) 2449-2453. |
| [6] | J. Hu, M. Chen, X.S. Fang, L.M. Wu, Fabrication and application of inorganic hollow spheres, Chem. Soc. Rev. 40(2011) 5472-5491. |
| [7] | Y. Li, D.L. Li, J.C. Liu, Optical and gas sensing properties of mesoporous hollow ZnO microspheres fabricated via a solvothermal method, Chin. Chem. Lett. 26(2015) 304-308. |
| [8] | X.F. Lin, X. Li, D.W. Sun, One step synthesis and characterization of monodispersed Ag2S nanoparticles, Jilin Norm. Univ. J. (Nat. Sci. Ed.) 28(2007) 77-79. |
| [9] | F. Gao, Q.Y. Lu, D.Y. Zhao, Controllable assembly of ordered semiconductor Ag2S nanostructures, Nano Lett. 3(2003) 85-88. |
| [10] | Q.Y. Lu, F. Gao, D.Y. Zhao, Creation of a unique self-supported pattern of radially aligned semiconductor Ag2S nanorods, Angew. Chem. (Ⅰ)nt. Ed. 114(2002) 2012-2014. |
| [11] | X.G. Wen, S.H. Wang, Y.T. Xie, X.Y. Li, S.H. Yang, Low-temperature synthesis of single crystalline Ag2S nanowires on silver substrates, J. Phys. Chem. B 109(2005) 10100-10106. |
| [12] | H.J. Zhai, H.S. Wang, Ag2S morphology controllable via simple template-free solution route, Mater. Res. Bull. 43(2008) 2354-2360. |
| [13] | X.H. Yang, Q.S. Wu, Y.P. Ding, G.X. Zhang, Controlled synthesis and optical properties of the semiconductor Ag2S nanotubes, Rare Met. Mater. Eng. 35(2006) 959-962. |
| [14] | Y.Z. Sun, B.B. Zhou, P. Gao, H.C. Mu, L.M. Chu, Single-crystalline Ag2S hollow nanoparticles and their ordered arrays, J. Alloys Compd. 490(2010) L48-L51. |
| [15] | Q.Y. Lu, F. Gao, D.Y. Zhao, A template-free method for hollow Ag2S semiconductor with a novel quasi-network microstructure, Chem. Phys. Lett. 360(2002) 355-358. |
| [16] | L.H. Dong, Y. Chu, Y. Liu, L.L. Li, Synthesis of faceted and cubic Ag2S nanocrystals in aqueous solutions, J. Colloid (Ⅰ)nterface Sci. 317(2008) 485-492. |
| [17] | K. Sahraoui, N. Benramdane, M. Khadraoui, R. Miloua, C. Mathieu, Characterization of silver sulphide thin films prepared by spray pyrolysis using a new precursor silver chloride, Sens. Transd. 27(2014) 319-325. |
| [18] | C.Z. Zhu, D. Du, A. Eychmüller, Y.H. Lin, Engineering ordered and nonordered porous noble metal nanostructures:synthesis, assembly, and their applications in electrochemistry, Chem. Rev. 115(2015) 8896-8943. |
| [19] | Y.X. Sun, D.W. Zhang, Z.W. Jin, Preparation and Application of Nanomaterials, China Textile & Apparel Press, Beijing, 2010. |
| [20] | S. Ahmad, A.A. (Ⅰ)sab, H.P. Perzanowski, Silver((Ⅰ)) complexes of thiourea, Trans. Met. Chem. 27(2002) 782-785. |
| [21] | P. Bombicz, (Ⅰ). Mutikainen,M. Krunks, et al., Synthesis, vibrational spectra and X-ray structures of copper((Ⅰ)) thiourea complexes, (Ⅰ)norg. Chim. Acta 357(2004) 513-525. |
| [22] | J.G. Yu, H. Guo, S.A. Davis, S.Mann, Fabrication of hollowinorganic microspheres by chemically induced self-transformation, Adv. Funct. Mater. 16(2006) 2035-2041. |
 2016, Vol.27
2016, Vol.27 


