b. School of Pharmacy, Jilin University, Changchun 130021, China
Bolaamphiphiles are a novel type of surfactants with two hydrophilic polar head groups linked via a single or multiple hydrophobic alkyl chains, which are drastically different from the conventional single head surfactants [1]. The special structure of bolaamphiphiles allows them arrange into mono-layer membrane (MLM) in aqueous solution to form vesicles that can be applied in drug delivery [2, 3]. Compared with the conventional bilayer liposome membrane (BLM) vesicles, MLM vesicles made with bolaamphiphiles are more stable [4]. This is because BLM vesicles may grow by fusion, while fusion is hindered in MLM vesicles due to the energy barrier caused by the difficulty encountered by the polar head groups in the process of crossing the hydrophobic membrane. This energy barrier also reduces the amphiphile exchange between vesicles and biological surfaces. In addition, the MLM vesicles of bolaamphiphiles have higher encapsulation capacity because of the larger inner aqueous core [5], and better controllable release behaviors as a result of the ability to switch from a vesicular structure to nano-ribbons, nanotubes or cylinders [6]. These advantages have made MLM vesicles of bolaamphiphiles excellent candidates in a number of paramount applications, such as biomimicking, controllable or targeted drug delivery, and imaging. Some research had been indicated that the synthetic bolaamphiphile molecules can form vesicles, which proved their ability of self-assembly, while more bolaamphiphiles inclined to form other structures such as fibers or liquid crystals [4, 6]. Due to the highly symmetric structure, it is a challenge for the synthetic bolaamphiphiles to form vesicle when applied as the drug carriers. Recently, some research had reported that it was more easily to form vesicles through the mixture of cholesterol [7] or other type surfactants [3] than that of the single one, herein bolaamphiphiles are promising nonconventional surfactants exploited in mixed surfactant formulations in drug delivery.
The hydrophilic parts of bolaamphiphile play a key role in fabricating functional MLM vesicles. For this reason, various functional groups were chosen as the hydrophilic part, such as fatty acids [8], thiophenes [9], and aza-crown-ethers [10]. Recently, peptidic bolaamphiphiles using amino acids [11, 12] as polar group have acquired more popularity. These molecules demonstrate a marked ability for self-organization in bulk solutions to form vesicles by adopting the self-assembly principles of amino acids while maintaining the characteristics of the oligopeptides [13]. This makes the peptidic bolaamphiphiles biocompatible, and the amino and carboxyl groups also endow these amphiphiles pH responsiveness. In this regard, the MLM vesicles made with the peptidic bolaamphiphiles are potential carriers for targeted drug delivery [14]. Both hydrophilic and hydrophobic drugs can be loaded in the spherical MLM vesicles [15, 16], and drug release may occur in acidic environments as a result of protonation of the amino group that increases the surfactants’ solubility and leads to the transformation of vesicle to other structures [17]. Since the pH value in tumor cells is low, the pH regulated drug release is expected to have great potential in cancer treatment.
In the present study, a novel peptidic bolaamphiphile, N,N'- (octane-1,8-diyl)bis(pyrrolidine-2-carboxamide) (DAOP), was designed and synthesized from proline and 1,8-diaminooctane, as the hydrophilic part and hydrophobic part, respectively. Proline is one of the twenty essential amino acids, which is critical in the construction of human bone and enamel. The structures of the synthesized bola surfactants were verified by MS, FTIR and 1HNMR. The pKa was measured by the acid-base titration method, the turbidity measurement was carried out using a Shimadzu UV-1750 spectrophotometer at 660 nm, and the critical micelle concentration (CMC) in water was determined by conductivity and fluorescence probe measurements. The vesicles of DAOP could be potential pH sensitive drug carriers.
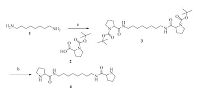
2. Experimental 2.1. Synthesis of the proline-based bolaamphiphileThe bolaamphiphile, N,N'-(octane-1,8-diyl)bis(pyrrolidine-2-carboxamide) (DAOP), was synthesized following the procedure in Scheme 1. Firstly, 1,8-diaminooctane (1) and Boc-protected proline (2) reacted under alkaline conditions in the presence of the catalyst [(Benzotriazol-1-yloxy)tris(dimethylamino)phosphoniumhexafluophosphate( BOP)] to obtain compound 3. The compound 4 was prepared by the removing of the protective group (Boc) in HCl [18, 19]. The details of the synthesis are given below:
|
Download:
|
| Scheme 1.The synthesis route of DAOP. Condition and regent: (a) BOP, DIEA, 22 ℃, 4 h, 95.7%; (b) HCl, r.t., 2 h, 95.1%. | |
The synthesis of compounds 3: 1,8-Diaminooctane (0.288 g, 2 mmol) was stirred and dispersed in 10 mL of dry dichloromethane followed by the addition of ethyldiisopropylamine (DIEA, 0.83 mL, 5 mmol), BOP (2.360 g, 5 mmol). The temperature was maintained at 22 ℃ while Boc-Pro-OH (1.071 g, 5 mmol) was dissolved in 5 mL of dry dichloromethane and added to the solution. The mixture was gradually dissolved under magnetic stirring. After 4 h, the solution was allowed to cool down to room temperature. The solvent was removed at reduced pressure, and 16 mL of ethyl acetate was added. The solvent was extracted with 20 mL of distilled water, 20 mL of saturated sodium bicarbonate and 20 mL of saturated sodium chloride solution. The ethyl acetate solution was dried overnight with anhydrous sodium sulfate. By evaporation under vacuum, yellow oil was obtained (1.64 g, yield: 95.7%).
The synthesis of compounds 4: The above bolaamphiphile precursor (0.646 g, 1.2 mmol) was stirred and dissolved in about 10 mL of dry ethyl acetate. A white solid precipitated out of solution by bubbling dry hydrogen chloride and isolated by filtration. Precipitation was dissolved in anhydrous methanol and dry hydrogen chloride was bubbled until N-Boc protection of amines was removed completely. Yellow oil was obtained after the solution was removed at reduced pressure (0.38 g, yield: 95.1%).
2.2. Physicochemical characterization of the DAOPThe pKa was measured by the acid-base titration method. The turbidity measurement was carried out using a Shimadzu UV-1750 spectrophotometer at 660 nm. The DAOP vesicles were prepared at different pH from 6.0 to 9.0 in the same DAOP concentration (18 mmol/L) [20, 21]. The critical micelle concentration (CMC) of DAOP in water was determined by conductivity and pyrene fluorescence spectroscopy. The pyrene was dissolved in ethanol and about 6 × 10-4 mmol pyrene was added to the sample tube. Then ethanol was removed under reduced pressure for 4 h and 2 mL of varying concentration surfactant was added to the tube. The mixture was ultrasounded for 3 h and incubated for 24 h and then the fluorescence absorbance was measured. Fluorescence absorbance measurements were taken by a multifunctional microplate reader (Mx3000P, ThermoScientific) using 12 mm path length quartz cuvettes. Excitation was done at 334 nm and emissions were recorded at 372 nm and 385 nm. The slit widths for both excitation and emission were fixed at 0.5 nm. Conductivity measurements were carried out at 25.0 ℃ using a DDS-11A conductivity meter (REX, Shanghai). In double distilled water, the DAOP vesicles self-assembled with a hydrophobic shell and hydrophilic core. Morphology of DAOP micelles was characterized by transmission electron microscopy (TEM, JEM-100CXII, JEOL, Japan). Particle size distribution was recorded by a Particle Size Analyzer (3000 HAS, Malvern).
3. Results and discussion 3.1. The characterization of DAOPThe structure of DAOP was characterized by IR, MS and 1H NMR. 1H NMR (600 MHz, DMSO-d6): δ 9.72 (s, 2H), 4.10 (t, 2H, J = 7.4 Hz), 3.22 (dd, 2H, J = 16.7, 11.3 Hz), 3.19-3.15 (m, 2H), 3.14-3.08 (m, 4H), 2.27 (qd, 3H, J = 14.4, 7.4 Hz), 1.88 (td, 5H, J = 14.2, 7.1 Hz), 1.78 (dt, 2H, J = 20.4, 7.5 Hz), 1.47-1.37 (m, 4H), 1.31-1.20 (m, 10H). IR (KBr, cm-1): 3421 (NH2 and CONH, υ(N-H)), 1671 (CONH, υ(C=O)), 1632 (NH2, δ(N-H)), 2923 (CH2, υs(C-H)), 2857 (CH2, υa(C-H)), 748 (CH2, ρ(C-H)), 1456 (CH2, δa(C-H)), 10= and 1032 (CH2, υ(C-C)). The mass spectrum showed a molecular weight of 339.3 as [M+H]+, consistent with the predicted 338.3.
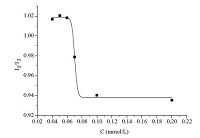
3.2. Physicochemical properties of DAOPThe value of CMC in water was 0.073 mmol/L, measured by the fluorescence probe method (Fig. 1) at 25 ℃, and the conductivity was proved 0.042 mmol/L (Fig. 2). Both results are far below the CMC values of conventional surfactants with comparable chain lengths (the reported CMC is 2.20-2.70 mmol/L for AOT and around 8 mmol/L for SDS at 25 ℃ [22, 23]), suggesting the strong self-assembling ability of the bolaamphiphile. The results of the two methods have a minor difference. The reason may be that the conductivity method was not sufficiently precise due to the lack of a sharp kink. As shown in Fig. 2, the variation of the electrical conductivity with the concentration does not show any clear break point around the CMC. The fluorescence probe method was considered to be more accurate for the fluorescence intensity ratio I1/I3 is very sensitive to the environment that the pyrene molecules are in. The environment of pyrene is more hydrophobic when the I1/I3 ratio decreases. The I1/I3 ratio is influenced not only by the solvent polarity but also by the aggregation number and core cavity.
|
Download:
|
| Fig. 1.I1/I3 vs. concentration profile for pyrene. The sigmoid line corresponds to the SBE fitting. | |

|
Download:
|
| Fig. 2.Dependence of the conductivity on the concentration of the surfactant at 25 ℃. | |
According to the structures, DAOP was amphiphilic with a hydrophobic C8 segment and two hydrophilic proline segments. In double distilled water, bolaamphiphilic molecules were inclined to assemble to monolayers. TEM images showed that DAOP vesicles were spherical vesicles with about 20 nm in diameter (Fig. 3), which was consistent to that measured by dynamic light scattering (DLS) with an average particle size of 29 nm. The sizes of vesicles are in the range that gives the tumor enhanced permeability and retention (EPR) effect [24]. Most solid tumors are different from normal tissues. According to the EPR effect, the blood vessel wall in tumor tissues becomes more permeable than in normal tissues. As a result, large molecules and even certain particles ranging from 10 nm to 200 nm in sizes, could go in and accumulate inside the interstitial space. And the nano particles may stay longer due to the absence of lymphatic drainage system in tumor tissues. In our study, the average particle size of DAOP vesicles was around 30 nm, which is a perfect size to bring drugs into tumors, especially macromolecular agents and drug-loaded nano carriers.

|
Download:
|
| Fig. 3.TEM photograph of DAOP vesicles (C = 18 mmol/L, 25 ℃). | |
Some research had investigated the toxicity of bolaamphiphiles that had the similar structures to that of DAOP. Khan et al. [12] had reported a novel bolaamphiphile as a gene delivery carrier using pentaethylenehexamine as the hydrophilicamine unit and 1,12- diaminododecane as the hydrophobic component, and the MTT assay proved it had no significant cytotoxicity in HEK293, HepG2, HeLa and 4T1 cells. Yang et al. [25] reported that a serial of bolaamphiphiles showed no cell toxicity for gene transfection whose hydrophilic part was lysine or glycine. In this study, it is estimated that DAOP will have no cell toxicity due to the structural similarity to the two reported surfactants.
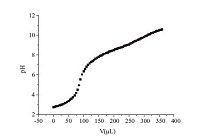
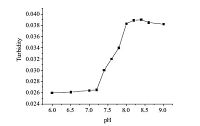
According to the pH titration experiment, the pKa value of DAOP was about 3.4 and 7.2 (Fig. 4). When the pH is below its pKa, the amino group will be protonated and is hard to form self-assembled structures in solutions. When the pH is above the pKa, the molecules are uncharged and are easy to assemble. In order to confirm the above hypothesis, the experiment of turbidity at pH value from 6.0 to 9.0 was conducted. The vesicles of DAOP at different pH (6-9) were prepared in the concentration of 18 mmol/L. The results of the turbidity measurements are shown in Fig. 5. Apparent increase in turbidity was observed as pH increased from 7.2 to 8.0 at room temperature, indicating the growth of the aggregates in the system. No phase separation or precipitate was observed during the transition. The result of turbidity was consistent with that of the pKa measurement. The pH value of tumor tissue is about 5.5-6.5, which is far below the pKa value (7.2) in normal tissues. Therefore, when pH is below the normal blood pH (7.4), the hydrophilic parts will be protonated and the repulsion of the ionic headgroups will lead to the vesicles rupture and drug release from vehicles. Another mechanism may be that the amide bonds formed by the reaction of amino acids and alkyl diamines will combine with the ions. As the pH decrease, it will lead to the breakage of the amide bonds, thus to release the drug. The premature drug release of vesicles was a concern with increased ionic strength, the pH of the surroundings and the dilution of body fluids. While the mainly self-assemble forces of DAOP vesicles involved hydrogen bonds and hydrophobic interactions, its pH-sensitivity is better than the liposome or micelles in the presence of serum. To study the leakage in the serum at neutral pH, a self-assemble procedure of DAOP vesicles was performed when the pH value is 7.2-7.4. Since the normal blood pH was 7.4 in a stable state, and will not fluctuate much in the body fluids, the vesicles will be stable in the pH range of serum. A predicted mechanism may be that the vehicles changed into micelles with the lower pH, which remained to be identified. Considering the pH sensitive property of DAOP, it could be applied as a pH-sensitive carrier for targeting on tumor tissues.
|
Download:
|
| Fig. 4.The pH value variation of DAOP as the adding of NaOH. | |
|
Download:
|
| Fig. 5.Turbidity curve of DAOP as a function of pH (C = 18 mmol/L, 25 ℃). | |
In summary, a novel proline-based bola surfactants were synthesized by a two-step sequence from Boc-proline and characterized by 1H NMR, FTIR and MS. The values of pKa and turbidity indicated that DAOP was a pH-sensitive bolaamphiphile. The low value of CMC benefits the formation of the vesicles. The DAOP vesicles have good morphology. The appearance of vesicles was translucent without suspended material, and the average particle size was about 30 nm. Our results indicate that the novel bolaamphiphile derived from proline has a potential to be an excellent drug carrier.
AcknowledgmentsThis work was supported by National Natural Science Foundation of China for Youth (No. 81202481), Education Department Foundation of Liaoning Province (No. L201=27), and Scientific Research Foundation for the Returned Overseas Scholars by State Education Ministry and by Shenyang Pharmaceutical University (No. GGJJ2014102).
| [1] | N. Nuraje, H. Bai, K. Su, Bolaamphiphilic molecules:assembly and applications, Prog. Polym. Sci. 38(2013) 302-343. |
| [2] | S. Landsmann, M. Luka, S. Polarz, Bolaform surfactants with polyoxometalate head groups and their assembly into ultra-small monolayer membrane vesicles, Nat. Commun. 3(2012) 1299. |
| [3] | Y. Yan, T. Lu, J. Huang, Recent advances in the mixed systems of bolaamphiphiles and oppositely charged conventional surfactants, J. Colloid (Ⅰ)nterface Sci. 337(2009) 1-10. |
| [4] | K. Liu, Y. Yao, C. Wang, et al., From bola-amphiphiles to supra-amphiphiles:the transformation from two-dimensional nanosheets into one-dimensional nanofibers with tunable-packing fashion of n-type chromophores, Chemistry 18(2012) 8622-8628. |
| [5] | T. Hutter, C. Linder, E. Heldman, S. Grinberg, (Ⅰ)nterfacial and self-assembly properties of bolaamphiphilic compounds derived from a multifunctional oil, J. Colloid (Ⅰ)nterface Sci. 365(2012) 53-62. |
| [6] | J. Huang, S. Wang, G. Wu, et al., Mono-molecule-layer nano-ribbons formed by self-assembly of bolaamphiphiles, Soft Matter 10(2014) 1018. |
| [7] | G.P. Kumar, P. Rajeshwarrao, Nonionic surfactant vesicular systems for effective drug delivery-an overview, Acta Pharm. Sin. B 1(2011) 208-219. |
| [8] | K.D. Danov, R.D. Stanimirova, P.A. Kralchevsky, et al., Sulfonated methyl esters of fatty acids in aqueous solutions:interfacial and micellar properties, J. Colloid (Ⅰ)nterface Sci. 457(2015) 307-318. |
| [9] | S.B. Yi, H.F. Gao, Q. Li, et al., Synthesis and self-assembly behavior of 2,5-diphenylethynyl thiophene based bolaamphiphiles, Chin. Chem. Lett. 26(2015) 872-876. |
| [10] | R. Muzzalupo, F.P. Nicoletta, S. Trombino, et al., A new crown ether as vesicular carrier for 5-fluoruracil:synthesis, characterization and drug delivery evaluation, Colloid Surf. B 58(2007) 197-202. |
| [11] | J. Kwak, S.Y. Lee, Enhanced photoluminescence by tyrosine-containing bolaamphiphile self-assembly, Langmuir 29(2013) 4477-4484. |
| [12] | M. Khan, C.Y. Ang, N. Wiradharma, et al., Diaminododecane-based cationic bolaamphiphile as a non-viral gene delivery carrier, Biomaterials 33(2012) 4673-4680. |
| [13] | C. Valery, F. Artzner, M. Paternostre, Peptide nanotubes:molecular organisations, self-assembly mechanisms and applications, Soft Matter 7(2011) 9583-9594. |
| [14] | M. Carafa, L. Di Marzio, C. Marianecci, et al., Designing novel pH-sensitive nonphospholipid vesicle:characterization and cell interaction, Eur. J. Pharm. Sci. 28(2006) 385-393. |
| [15] | D.R. Nogueira, L.E. Scheeren, M. Pilar Vinardell, et al., Nanoparticles incorporating pH-responsive surfactants as a viable approach to improve the intracellular drug delivery, Mater. Sci. Eng. C-Mater. 57(2015) 100-106. |
| [16] | J. Kwak, S.Y. Lee, pH-sensitive transformation of the peptidic bolaamphiphile selfassembly:exploitation for the pH-triggered chemical reaction, Colloid Surf. B 115(2014) 406-411. |
| [17] | R. Bordes, K. Holmberg, Amino acid-based surfactants-do they deserve more attention? Adv. Colloid (Ⅰ)nterface 222(2015) 79-91. |
| [18] | J.R. Yao, D.H. Xiao, X. Chen, et al., Synthesis and solid-state secondary structure investigation of silk-proteinlike multiblock polymers, Macromolecules 36(2003) 7508-7512. |
| [19] | J. Xu, S. Chen, G. Tang, X. Wang, The synthesis of amide dendritic gelators and its self-assembly behavior in MMA, J. Macromol. Sci. A 48(2011) 896-903. |
| [20] | H. Yin, Z. Zhou, J. Huang, R. Zheng, Y. Zhang, Temperature-induced micelle to vesicle transition in the sodium dodecylsulfate/dodecyltriethylammonium bromide system, Angew. Chem. (Ⅰ)nt. Ed. 42(2003) 2188-2191. |
| [21] | J. Zhang, L. Bing, G.A. Reineccius, Comparison of modified starch and Quillaja saponins in the formation and stabilization of flavor nanoemulsions, Food Chem. 192(2016) 53-59. |
| [22] | A.(Ⅰ). Mitsionis, T.C. Vaimakis, Estimation of AOT and SDS CMC in a methanol using conductometry, viscometry and pyrene fluorescence spectroscopy methods, Chem. Phys. Lett. 547(2012) 110-113. |
| [23] | E. Acosta, M. Bisceglia, J.C. Fernandez, Electric birefringence of AOT/water solutions, Colloid Surf. A 161(2000) 417-422. |
| [24] | V. Torchilin, Tumor delivery of macromolecular drugs based on the EPR effect, Adv. Drug Deliv. Rev. 63(2011) 131-135. |
| [25] | P. Yang, J. Singh, S. Wettig, et al., Enhanced gene expression in epithelial cells transfected with amino acid-substituted gemini nanoparticles, Eur. J. Pharm. Biopharm. 75(2010) 311-320. |
 2016, Vol.27
2016, Vol.27 


