b. Center of Analysis and Measurement, Anhui Science and Technology University, Fengyang 233100, China
Since the report of organic light-emitting diodes (OLED) based on phosphorescent emitters, there has been intensive interest in these devices because they can harvest both singlet and triplet excitons for emission, providing the opportunity to realize internal quantum efficiency close to 100% [1]. Until now, most of the efficient phosphorescent organic light-emitting diodes (PHOLEDs) have been fabricated through vacuum thermal evaporation which allows for complicated vertical device architectures and delivers excellent devices with high efficiencies [2, 3], but requires complicated technological processes and, consequently, is much more costly. It is generally believed that solution-based processes, such as spin-coating or ink-jet printing, are relatively inexpensive and can be utilized for the preparation of large-area displays [4]. To date, solution-processed PHOLEDs are mainly focused on polymeric materials by incorporation of heavy metal complexes through physical blending in polymer hosts like polyvinylcarbazole (PVK) [5] and polyfluorene (PFO) [6] or chemical bonding on polymer chains [7]. However, the applicability of polymeric materials is greatly hampered by intrinsic disadvantages, such as the uncertain molecular structure and difficulty in purification, while the purity of materials has a great influence on electroluminescence performance. In contrast, conjugated dendrimers have emerged as a new type of light-emitting organic materials for application in OLEDs [8]. They combine the advantages of both small molecules and polymeric materials, for example, repeatable monodispersity and high levels of purity of small molecules, and good solubility and film-formation ability by wet methods of polymers [9, 10]. Therefore, dendritic molecules with good solubility and excellent thermal stability, either as hosts or doped emitters for solution-processible phosphorescent light-emitting devices, have been investigated [11, 12].
Due to the relatively long excited state lifetimes of triplet emitters, the heavy metal complexes are usually doped into host materials to reduce the self-quenching and triplet-triplet annihilation [13]. By the combination of both hole and electron transporting moieties in one molecule, bipolar molecules in the quest for highly efficient PHOLEDs have aroused considerable interest because they enable a balanced density of charge and simplified device structures [14, 15]. One of the most important core structures of bipolar host molecules is the carbazole moiety due to its large triplet energy and good hole-transport property [16, 17, 18, 19, 20, 21]. To achieve bipolarity in the molecular designs based on carbazole, various moieties capable of accepting electrons were incorporated to give novel bipolar hosts [22, 23, 24]. For example, Yang et al. reported a bipolar dendrimer with carbazole units as hole-transporting units and oxadiazole units as electron-transporting units with maximum efficiencies of 16.8 cd A-1 and 5.7% obtained for solution-processed green PHOLEDs by using Ir(ppy)3 as guests [23]. Most recently, Li et al. reported a carbazole-based molecule as a universal bipolar host material by attaching 3,6- bis(3,6-di-tert-butyl-carbazol-9-yl)-carbazole and pyrazole to the dimethylbiphenyl core, achieving high luminance efficiencies of 35 cd A-1 and 39 cd A-1 for green and red electrophosphorescence, respectively, by incorporating electron-transporting 2, 2'-(1,3- phenylene)bis[5-(4-tert-butylphenyl)-1,3,4-oxadiazole] (OXD-7) in the emitting layer as a mixed host [24].
In this paper, we report the synthesis and characterization of two novel carbazole-based dendrimers, namely G1SF and G2SF, for potential application as bipolar host materials. The electronaccepting dibenzothiophene was selected as the core, and the wellknown electron-donating oligo-carbazole dendrons were attached at both terminals of the dibenzothiophene. Furthermore, tert-butyl groups are introduced into the dendrimers surface to enhance the solubility of these dendrimers. The dendrimers designed in this way are observed to exhibit excellent thermal stability, to possess relatively shallow, highest occupied, molecular orbital (HOMO) levels, and to have the spatial separation of the HOMO and LUMO energy levels. The green PHOLEDs using G1SF or G2SF, as a host material by the spin-coating method with traditional Ir(ppy)3 as doped emitter, show the maximum luminance efficiency (ηL) of 19.83 cd A-1 and a maximum external quantum efficiency of 5.85%.
2. Experimental 2.1. Materials and methodsChemicals, reagents and solvents from commercial sources are of analytical, or spectroscopy grade and used as received without further purification. The 1H NMR spectra were recorded on an AVANCE-400 NMR spectrometer (400 MHz). Mass spectra were recorded on a gas chromatograph-time of flight (GC-TOF) mass spectrometer (Micromass, UK) for EI-TOF-MS and a MALDI micro MX (Waters, USA) for matrix-assisted laser desorption time of flight (MALDI-TOF) mass spectra. Thermogravimetry analyses (TGA) were carried out using a Pyris1 TGA (PerkinElmer Corp., USA) at a heating rate of 10 ℃ min-1 under a nitrogen atmosphere. The fluorescence and UV-vis absorption spectra measurements were performed on a Hitachi F-4600 spectrofluorophotometer and a UV- 265 spectrophotometer, respectively. Electrochemical measurements were made by using a conventional three-electrode configuration and an EG & G PAR 283 potentiostatic instrument at a scan rate of 100 mV s-1. A glassy carbon working electrode, a Pt-wire counter electrode, and a saturated calomel electrode (SCE) as reference electrode were used. All measurements were made at r.t. on samples dissolved in dichloromethane, with 0.1 mol L-1 tetra-n-butyl ammonium hexafluorophosphate (Bu4NPF6) as the electrolyte, and ferrocene as the internal standard [25]. Density functional theory (DFT) calculations using B3LYP functional were performed. The basis set used for C, H, N atoms was 6-31G. There are no imaginary frequencies for both optimized structures. All these calculations were performed with Gaussian 09 [26].
The patterned ITO substrates were cleaned by successive ultrasonications in detergent, deionized water, ethanol, and dichloromethane, followed by treatment with UV-ozone for 20 min. A 40 nm thick PEDOT:PSS film was first spin-coated on pre-treated ITO substrates from an aqueous dispersion and baked at 120 ℃ for 40 min in air. Subsequently, blends of host: Ir(ppy)3 in chlorobenzene were filtered through a 0.45 mm PTFE filter and spin-coated on PEDOT/PSS film, the thickness of which was controlled at 40 nm by adjusting the spin rate. The substrate was transferred into a vacuum chamber to deposit the TPBI layer at a base pressure less than 10-6 Torr. Finally, the device fabrication was completed through thermal deposition of LiF (1 nm) and then capping with Al metal (100 nm) as cathode. The EL spectra, CIE coordinates, and current density-voltage-luminance relationships of devices were measured by the JY SPEX CCD 3000 photometer and a Keithley 237 instrument. All the measurements were carried out in ambient condition at r.t.
2.2. Synthesis and characterizationThe carbazole dendrons D1 and D2 were synthesized and characterized according to literature methods [27].
Synthesis of compound 1: To a solution of dibenzothiophene (940 mg, 5.5 mmol), iodine (1.34 g, 5.2 mmol), iodic acid (1.58 g, 9.0 mmol), acetic acid (5 mL) and chloroform (5 mL), was added water (0.5 mL) and concentrated sulfuric acid (0.5 mL, 98%). The mixture was stirred at 50 ℃ for 18 h, and then 10 mL water was added. The organic layer was separated and washed with diluted HCl and brine, then dried over anhydrous MgSO4. The solvent was removed under vacuum and the residue was recrystallized from a mixed solvent of chloroform/ethanol (v/v = 3:1) to give 1 as a lightyellow crystal (1.83 g, 75% yield). EI-TOF-MS (m/z): 435.84 [M+].
Synthesis of G1SF: A mixture of compound 1 (100 mg, 0.23 mmol), D1 (154 mg, 0.55 mmol), CuI (5 mg, 0.03 mol), and K2CO3 (20 mg, 0.15 mmol) were added to a 50 mL 2-neck flask, and then 18-crown-6 (5 mg, 0.02 mmol) and o-dichlorobenzene (20 mL) were added under a nitrogen atmosphere. After stirring for 12 h at 160 ℃, the reaction mixture was cooled to r.t. The solvent was removed under reduced pressure, dichloromethane and water were added. The organic layer was separated and washed with diluted HCl and brine, then dried over anhydrous MgSO4. The solvent was removed to dryness and the residue was purified by column chromatography over silica gel with petroleum ether/dichloromethane (4:1) as the eluent to give G1SF as a white solid (224 mg, 60% yield). Confirmation data: 1H NMR (400 MHz, CDCl3): δ 8.28 (d, 2H, ArH), 8.15 (d, 4H, ArH), 8.10 (s, 1H, ArH), 8.08 (s, 1H, ArH), 7.71-7.69 (m, 2H, ArH), 7.46-7.43 (m, 4H, ArH), 7.37 (s, 2H, ArH), 7.34 (s, 2H, ArH), 1.45 (s, 36H, CH3); MALDI-TOF-MS (m/z): calcd. for C52H54N2S, 738.4008; found: 738.3795 [M+].
Synthesis of G2SF: A mixture of compound 1 (100 mg, 0.23 mmol), D2 (400 mg, 0.55 mmol), CuI (5 mg, 0.03 mmol), and K2CO3 (20 mg, 0.15 mmol) were added to a 50 mL 2-neck flask, and then 1, 10-phenanthroline monohydrate (10 mg, 0.05 mmol) and toluene (20 mL) were added under a nitrogen atmosphere. After stirring for 48 h at 110 ℃, the solvent was removed under reduced pressure. Water was added and the mixture was extracted with dichloromethane. The organic layer was separated and washed with diluted HCl and brine, then dried over anhydrous MgSO4. The solvent was removed to dryness and the residue was purified by column chromatography over silica gel with petroleum ether/dichloromethane (4:1) as the eluent to give G2SF as a white solid (58 mg, 35% yield). Confirmation data: 1H NMR (400 MHz, CDCl3): δ 8.56 (s, 2H, ArH), 8.29-8.24 (m, 6H, ArH), 8.13 (s, 8H, ArH), 7.92-7.89 (m, 2H, ArH), 7.64-7.57 (m, 8H, ArH), 7.43-7.41 (m, 8H, ArH), 7.32 (d, 8H, ArH), 1.45 (s, 72H, CH3); MALDI-TOF-MS (m/z): calcd. for C116H114N6S, 1624.2516; found: 1624.0037 [M+].
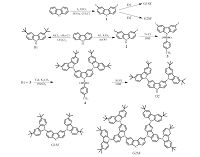
3. Results and discussionThe synthetic routes for the target dendrimers (G1SF, G2SF) are shown in Scheme 1. First, the important intermediates including the carbazole dendrons (D1, D2) were synthesized according to literature methods [27], as detailed in Scheme 1. The 3,6-diiododibenzothiophene (1) was then obtained at a high yield of 75% by iodination of benzothiophene in presence of iodic acid at 50 ℃. It is found that use of the traditional periodic acid often resulted in mono-iodinated byproducts. Finally, the target compounds G1SF and G2SF were then prepared at a moderate yield of 35% and 60% by the Ullmann C-N coupling reaction between the bis-iodinated dibenzothiophene 1 with an excess molar ratio of carbazole dendrons D1 and D2, respectively. They have good solubility in common organic solvents such as dichloromethane, tetrahydrofuran and ethyl acetate, so they can be easily purified by column chromatography and recrystallized to high purity for spectroscopic characterization and OLEDs application. Their chemical structures were confirmed by 1H NMR spectroscopy, MALDI-TOF mass spectrometry.
|
Download:
|
| Scheme 1.Structures and synthetic routes of G1SF and G2SF. | |
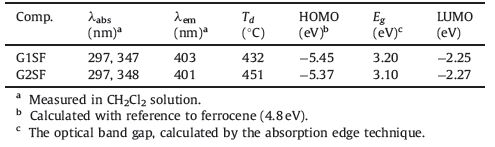
The thermal stability of the studied dendrimers was investigated by means of thermogravimetric analysis (TGA) at a scanning rate of 10 ℃ min-1 and the data are listed in Table 1. As shown by the TGA curves in Fig. 1, G1SF and G2SF exhibit high thermochemical stability with decomposition temperatures (Td, corresponding to 5% weight loss) up to 430 ℃. This is an essential feature for organic light-emitting materials especially when they are used at high temperature. The thermal stability is attributed to the presence of carbazole dendrons, which are known for their excellent thermal and chemical stabilities [28].
|
|
Table 1 Summary of physical data of the dendrimers. |

|
Download:
|
| Fig. 1.TGA traces of (a) G1SF and (b) G2SF. | |
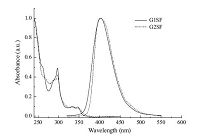
The photophysical properties of these dendrimers were investigated by means of electronic absorption and steady state photoluminescence (PL) measurements of dilute solutions in dichloromethane. The pertinent data are summarized in Table 1. As shown in Fig. 2, both dendrimers exhibit two major absorption bands in the ultra-violet region. The absorption around 297 nm could be assigned to the π-π* local electron transition of the carbazole dendrons at the terminal ends, and the longer wavelength ranging from 310 nm to 370 nm could be attributed to the π-π* electron transition of the entire conjugated backbone. Upon photoexcitation at the absorption maxima, both the dendrimers exhibit deep-blue emission in dichloromethane solutions with emission peaks at 403 nm and 401 nm for G1SF and G2SF. Furthermore, there is little overlap between the absorption and emission spectra for these two molecules, thus self-absorption can be dramatically eliminated, which is a very important attribute for materials used in optoelectronic devices.
|
Download:
|
| Fig. 2.Absorption and fluorescence spectra of G1SF and G2SF in dilute dichloromethane solutions. | |
The electrochemical behavior of these dendrimers was investigated by the cyclic voltammetry method in a nitrogen atmosphere, as shown in the cyclic voltammograms in Fig. 3. Upon the anodic sweep, G1SF and G2SF show one and two reversible oxidation processes, respectively, with the first oxidation wave of G2SF shifting to less positive potentials than that of G1SF. This implies that the removal of the first electron from these dendritic molecules become easier with increasing dendron generation. This is inconsistent with the previous observation that the electron density increasingly gathers on the outmost carbazole rings with increasing carbazole dendron generation [29]. The onset potential Eoxonset of the first oxidation wave is in the range of 0.65 × 0.57 V relative to ferrocene for these materials. The HOMO energy levels were estimated from the onset potentials to be-5.45 and-5.37 eV for G1SF and G2SF, respectively, according to the equation of HOMO (eV) = -(Eoxonset 4.8 eV) [24]. Apparently the HOMO levels of these carbazole-based dendrimers are higher than those of both CBP (-5.69 eV) and PVK (-5.8 eV) and quite close to the work function of the widely-used hole injecting material PEDOT:PSS (-5.2 eV) [30], implying that efficient hole injection into the emitting layer could be expected when these dendrimers are used as host materials in the emitting layer of PHOLEDs. Because no clear reduction wave was observed within the potential window of the cyclic voltammograms, the LUMO energy levels were deduced from HOMO energy levels and the optical band gaps determined by the onset of absorption [24]. The deduced LUMO levels are -2.25 eV and -2.27 eV for G1SF and G2SF, respectively. The detailed electrochemical and electronic data of these molecules are listed in Table 1.
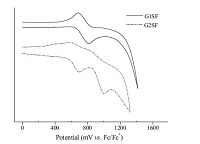
|
Download:
|
| Fig. 3.Cyclic voltammograms of G1SF and G2SF measured in CH2Cl2 at a scan rate of 100 mV s-1. | |
In order to gain insight into the electronic properties of these dendrimers, the spatial distribution of HOMO and LUMO was calculated with the Gaussian 03 package at the B3LYP/6-31G(d) level, using the Density Function Theory (DFT) for the geometry optimization [21]. Fig. 4 illustrates the HOMO and LUMO distribution for both G1SF and G2SF. It is obvious that, G1SF exhibits only a partial separation of the HOMO and LUMO levels, possibly due to intramolecular charge transfer. The HOMO of G1SF spreads over the whole molecular skeleton with major contributions from the two carbazole rings, while the LUMO is mainly located on the benzothiophene moiety. But for G2SF, it is observed that the HOMO orbital is mainly located on the electron-donating carbazole dendron moiety, while the LUMO orbital delocalize on the electron-accepting benzothiophene moiety. The separation between HOMO and LUMO levels is preferable for efficient holeand electron-transporting properties (bipolar) and the prevention of reverse energy transfer, and thus to benefit to the EL performance of the PHOLEDs.
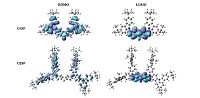
|
Download:
|
| Fig. 4.Frontier molecular orbitals (HOMO and LUMO) distribution for G1SF and G2SF calculated with DFT on a B3LYP/6-31G(d) level. | |
In order to evaluate the capability of G1SF and G2SF as host materials in PHOLEDs, the green-light emitting devices with the configuration of ITO/PEDOT:PSS (40 nm)/G1SF (device A) or G2SF (device B): Ir(ppy)3 (6 wt%, 40 nm)/TPBI (30 nm)/LiF (1 nm)/Al (100 nm) was fabricated. In these devices, PEDOT:PSS [poly(3,4- ethylenedioxythiophene):poly(styrene sulfonate)] is used as the hole-injecting layer. The Ir(ppy)3 doped emitting layer was fabricated by spin coating the mixed solution of the host and dopant on top of the PEDOT:PSS layer. The doping concentration was carefully tuned in order to reach as good a performance as possible. TPBI {1,3,5-tris[N-(phenyl)benzimidazole]-benzene} is then deposited by vacuum thermal evaporation on top of the emitting layer to act as the electron-transporting and holeblocking layer. Both devices show typical green emission at around 512 nm originating from the guest Ir(ppy)3, as show by the EL spectra in Fig. 5. The absence of any residual emission from the dendrimer hosts or TPBI layer even at high current densities suggests a complete energy transfer from these dendrimer hosts to the iridium dopant and the effective hole-blocking function of TPBI layer in addition to its electron-transporting role.
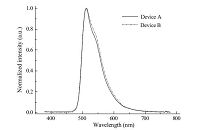
|
Download:
|
| Fig. 5.EL spectra of the green devices A and B at 10 V. | |
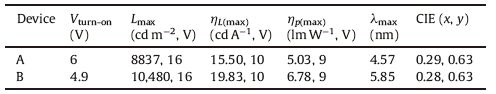
The current density-voltage-brightness (J-V-L) characteristics and efficiencies versus current density curves of the devices are shown in Fig. 6. The G2SF based device B has a lower turn-on voltage (4.9 V, corresponding to 1 cd m-2) than that (6.0 V) for G1SF based device A. It is also evident that the current density of device B was higher than that of the corresponding device A in spite of the identical device configuration, presumably because of the facilitated hole injection and transportation in device B. According to the energy level diagram in Fig. 7, the HOMO level of G2SF (-5.37 eV) is slightly higher than that of G1SF (-5.45 eV). Thus the slightly smaller hole injection barrier at the ITO/dendrimer interface in G2SF based device B should be responsible for its lower driving voltages. In addition, the possible high hole-transporting mobility in the bulky layer of dendrimer G2SF due to high density of hole-transporting carbazole groups is probably another reason for the relatively high current in G2SF based device B. The G1SF based device A exhibited a maximum luminance of 8837 cd m-2 at 16 V, and a maximum luminance efficiencyηLof 15.50 cd A-1, corresponding to a peak power efficiency ηp of 5.03 lm W-1 and forward-viewing external quantum efficiency ηext of 4.57%. The G2SF based device B achieved the maximum efficiencies of 19.83 cd A-1, 6.78 lm W-1 and 5.85%. Apparently, the efficiencies of the present devices exceed the best literature data (15 cd A-1) of the partially solution-processed phosphorescent device with PVK:Ir(ppy)3 emitting layer [31]. The detailed performances of these devices are summarized in Table 2.

|
Download:
|
| Fig. 6.(a) Voltage-current density-luminance (V-J-L) characteristics and (b) the luminance efficiency and external quantum efficiency curves for the green-emitting devices A and B. | |

|
Download:
|
| Fig. 7.Energy level diagram for the OLEDs. Insert: chemical structures of Ir(ppy)3. | |
|
|
Table 2 EL data of devices A and B. |
In summary, novel carbazole-based dendrimers have been developed by attaching oligo-carbazole dendrons at both terminals of the dibenzothiophene core through Ullmann coupling reactions. The thermochemical and electrochemical studies revealed that these compounds have high thermal stability with Td values of over 430 ℃ and excellent electrochemical reversibility. More importantly, these molecules have the spatial separation of the HOMO and LUMO energy levels at the electron donor and acceptor moieties. The solution-processed green electrophosphorescent devices by using these dendritic hosts and iridium complex dopant exhibited good performance with the maximum luminance efficiency (ηL) of 19.83 cd A-1 and a maximum external quantum efficiency of 5.85%. The present study demonstrated that these carbazole-based dendrimers may find potential applications as bipolar host materials for solution-processed PHOLEDs.
AcknowledgmentsWe thank the Natural Science Foundation of Anhui Province (Nos. KJ2013A079, KJ2016A184) and the Research Funds of Anhui Science and Technology University (Nos. AKZDXK2015A01, ZRC2014401, ZRC2014432) for financial support of this work.
| [1] | Y.T. Tao, C.L. Yang, J.Q. Qin, Organic host materials for phosphorescent organic light-emitting diodes, Chem. Soc. Rev. 40(2011) 2943-2970. |
| [2] | C.H. Chang, M.C. Kuo, W.C. Lin, et al., A dicarbazole-triazine hybrid bipolar host material for highly efficient green phosphorescent OLEDs, J. Mater. Chem. 22(2012) 3832-3838. |
| [3] | C.W. Lee, J.Y. Lee, High quantum efficiency and color stability in white phosphorescent organic light emitting diodes using a pyridine modified carbazole derivative, Dyes Pigments 103(2014) 34-38. |
| [4] | M.R. Zhu, T.L. Ye, X.S. He, et al., Highly efficient solution-processed green and red electrophosphorescent devices enabled by small-molecule bipolar host material, J. Mater. Chem. 21(2011) 9326-9331. |
| [5] | C.Y. Jiang, W. Yang, J.B. Peng, S. Xiao, Y. Cao, High-efficiency, saturated redphosphorescent polymer light-emitting diodes based on conjugated and nonconjugated polymers doped with an (Ⅰ)r complex, Adv. Mater. 16(2004) 537-541. |
| [6] | J. Liu, Q.G. Zhou, Y.X. Cheng, et al., The first single polymer with simultaneous blue, green, and red emission for white electroluminescence, Adv. Mater. 17(2005) 2974-2978. |
| [7] | Z.H. Ma, J.Q. Ding, B.H. Zhang, et al., Red-emitting polyfluorenes grafted with quinoline-based iridium complex:"Simple polymeric chain, unexpected high efficiency", Adv. Funct. Mater. 20(2010) 138-146. |
| [8] | D. Liu, Y.H. Duan, Synthesis of novel thieno-[3,4-b]-pyrazine-cored molecules as red fluorescent materials, Chin. Chem. Lett. 24(2013) 809-812. |
| [9] | J.Y. Li, T. Zhang, Y.J. Liang, R.X. Yang, Solution-processible carbazole dendrimers as host materials for highly efficient phosphorescent organic light-emitting diodes, Adv. Funct. Mater. 23(2013) 619-628. |
| [10] | C. Huang, C.G. Zhen, S.P. Su, et al., High-efficiency solution processable electrophosphorescent iridium complexes bearing polyphenylphenyl dendron ligands, J. Organomet. Chem. 694(2009) 1317-1324. |
| [11] | J.H. Zou, H. Wu, C.S. Lam, et al., Simultaneous optimization of charge-carrier balance and luminous efficacy in highly efficient white polymer light-emitting devices, Adv. Mater. 23(2011) 2976-2980. |
| [12] | J.Q. Ding, J.H. Lü, Y.X. Cheng, Z.Y. Xie, L.X. Wang, Solution-processible red iridium dendrimers based on oligocarbazole host dendrons:synthesis, properties, and their applications in organic light-emitting diodes, Adv. Funct. Mater. 18(2008) 2754-2762. |
| [13] | Q.S. Zhang, D. Tsang, H. Kuwabara, et al., Nearly 100% internal quantum efficiency in undoped electroluminescent devices employing pure organic emitters, Adv. Mater. 27(2015) 2096-2100. |
| [14] | M.S. Lin, L.C. Chi, H.W. Chang, et al., A diarylborane-substituted carbazole as a universal bipolar host material for highly efficient electrophosphorescence devices, J. Mater. Chem. 22(2012) 870-876. |
| [15] | W.Y. Huang, T.C. Wang, H.C. Chiu, H.F. Chenb, K.T. Wong, A spiro-configured ambipolar host material for impressively efficient single-layer green electrophosphorescent devices, Phys. Chem. Chem. Phys. 12(2010) 10685-10687. |
| [16] | Y.T. Tao, Q. Wang, C.L. Yang, et al., A simple carbazole/oxadiazole hybrid molecule:an excellent bipolar host for green and red phosphorescent OLEDs, Angew. Chem. (Ⅰ)nt. Ed. Engl. 47(2008) 8104-8107. |
| [17] | Q. Peng, M.J. Li, S.Q. Lu, X.H. Tang, An efficient blue-emitting conjugated copolymer based on fluorene and carbazole with a peripheral dendritic carbazole pendant at the 9-position, Macromol. Rapid Commun. 28(2007) 785-791. |
| [18] | K.T. Wong, Y.H. Lin, H.H. Wu, F. Fungo, Synthesis and properties of dumbbellshaped dendrimers containing 9-phenylcarbazole dendrons, Org. Lett. 9(2007) 4531-4534. |
| [19] | C.W. Wu, C.M. Tsai, H.C. Lin, Synthesis and characterization of poly (fluorene)-based copolymers containing various 1,3,4-oxadiazole dendritic pendants, Macromolecules 39(2006) 4298-4305. |
| [20] | Q. Peng, J. Xu, M.J. Li, W.X. Zheng, Blue emitting polyfluorenes containing dendronized carbazole and oxadiazole pendants:synthesis, optical properties, and electroluminescent properties, Macromolecules 42(2009) 5478-5485. |
| [21] | X.D. Wang, S.M. Wang, Z.H. Ma, J.Q. Ding, Solution-processible 2,2'-dimethylbiphenyl cored carbazole dendrimers as universal hosts for efficient blue, green, and red phosphorescent OLEDs, Adv. Funct. Mater. 24(2014) 3413-3421. |
| [22] | M.K. Kim, J. Kwon, T.H. Kwon, J.(Ⅰ). Hong, A bipolar host containing 1,2,3-triazole for realizing highly efficient phosphorescent organic light-emitting diodes, New J. Chem. 34(2010) 1317-1322. |
| [23] | J. Yang, T.L. Ye, Q. Zhang, D.G. Ma, "Click" synthesis of a bipolar dendrimer as a host material for electrophosphorescent devices, Macromol. Chem. Phys. 211(2010) 1969-1976. |
| [24] | L.J. Deng, J.Y. Li, W. Li, Solution-processible small-molecular host materials for high-performance phosphorescent organic light-emitting diodes, Dyes Pigments 102(2014) 150-158. |
| [25] | R.J. Wang, L.J. Deng, M. Fu, J.L. Cheng, J.Y. Li, Novel ZnⅡ complexes of 2-(2-hydroxyphenyl) benzothiazoles ligands:electroluminescence and application as host materials for phosphorescent organic light-emitting diodes, J. Mater. Chem. 22(2012) 23454-23460. |
| [26] | Y.T. Tao, Q. Wang, C.L. Yang, et al., Solution-processable highly efficient yellowand red-emitting phosphorescent organic light emitting devices from a small molecule bipolar host and iridium complexes, J. Mater. Chem. 18(2008) 4091-4096. |
| [27] | H.Q. Zhang, S.M. Wang, Y.Q. Li, et al., Synthesis, characterization, and electroluminescent properties of star shaped donor-acceptor dendrimers with carbazole dendrons as peripheral branches and heterotriangulene as central core, Tetrahedron 65(2009) 4455-4463. |
| [28] | J.B. Yuan, Z.G. Zhang, L.M. Leung, K.L. Zhang, Synthesis and characterization of novel star-shaped pyridine cored compounds with alternating carbazole and triphenylamine moieties, Chin. Chem. Lett. 19(2008) 647-650. |
| [29] | K. Albrecht, K. Yamamoto, Dendritic structure having apotential gradient:new synthesis and properties of carbazole dendrimers, J. Am. Chem. Soc. 131(2009) 2244-2251. |
| [30] | N. Koch, A. Elschner, J.P. Rabe, R.L. Johnson, Work function independent holeinjection barriers between pentacene and conducting polymers, Adv. Mater. 17(2005) 330-335. |
| [31] | H.M. Lee, S.J. Baek, S.C. Gong, et al., Preparation and characterization of phosphorescence organic light-emitting diodes using poly-vinylcarbazole:tris (2-phenylpyridine) iridium (Ⅲ) emission layer, Opt. Eng. 48(2009) 104001. |
 2016, Vol.27
2016, Vol.27