b. Research Center of Energetic Material Genome Science, Institute of Chemical Materials, China Academy of Engineering Physics, Mianyang 621050, China
Energetic materials are used for various military and civilian purposes [1, 2]. Particularly, high-energy densitymaterials (HEDMs) have attracted considerable attention [3, 4, 5]. The preferred characteristics for HEDMs include high density, positive heat of formation, positive oxygen balance, low sensitivity, and etc. Some substituents, such as nitro group (NO2) and nitroamine group (NHNO2), play an important role in increasing the oxygen balance and density [6]. Therefore, the introduction of the nitro or nitroamine group into the energetic molecules is an efficient approach to improving the detonation performance of energetic materials. However, most of them are sensitive [7]. In order to solve the conflict of sensitivity and high energy, salification is an effective way. And thus prepared energetic salts usually possess some advantages such as low vapor pressure and low sensitivity [8].
Stability and sensitivity of energetic salts are influenced by cations and anions. We can take advantage of nitrogen-rich compounds bridge between anions and cations [9]. Among these anions, our group has recently focused on the 1,2,4-oxadiazole parent ring and synthesized its energetic derivatives with high energy, high thermal stability and low sensitivity [10, 11]. Herein, we reported a series of energetic salts derived from 3-nitro-5- nitroimino-1,2,4-oxadiazole (NON).
2. Experimental1H NMR and 13C NMR spectra were recorded on a BRUKER Advance 400 spectrometer at 400 and 100 MHz, respectively. IR spectra were carried out on an IR-408 or BRUKER Alpha using KBr pellets. Elemental analyses were measured using an Elemental Vario MICRO CUBE elemental analyzer. Melting and decomposition points were obtained by differential scanning calorimetry (DSC) on a METTLER Toledn apparatus at a scan rate of 10 ℃/min.
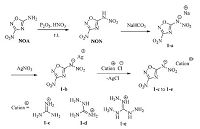
2.1. Preparation of 3-nitro-5-nitroimino-1,2,4-oxadiazole (NON) and its sodium salt 1-aP2O5 (5 g, 35 mmol) was solved in fuming HNO3 (6 mL) at -15 ℃, followed by the addition of 3-nitro-5-amino-1,2,4-oxadiazole (NOA) [10a] (260 mg, 2 mmol). The reaction mixture was stirred at room temperature for 12 h. Then the mixture was poured into ice water and the product NON was neutralized with solid NaHCO3 till pH 8.0. The sodium salt was extracted with ethyl acetate (50 mL × 4). The combined organic layers were dried over anhydrous Na2SO4. And the solvent was removed under reduced pressure to afford sodium salt (1-a) (296 mg, 75%) for the next step (Scheme 1).
|
Download:
|
| Scheme 1.Synthesis of 3-nitro-5-nitroimino-1,2,4-oxadiazole (NON) and salts of 1-c, 1-b, 1-e. | |
A solution of AgNO3 (340 mg, 2 mmol) in H2O (25 mL) was added dropwise to a solution of the sodium salt 1-a (394 mg, 2 mmol) in H2O (15 mL) upon stirring at room temperature. After 2 h stirring the precipitate was collected, washed with water and dried giving a yellow solid of silver salt 1-b (451 mg, 80%).
2.3. General procedure for synthesis of energetic salts 1-c, 1-d, and 1-eA solution of guanidinium chloride (1 mmol), aminoguanidinium chloride (1 mmol), or triaminoguanidinium chloride (1 mmol) in water (2 mL) was added dropwise to the suspension of precursor salt 1-b (0.5 mmol) in water (10 mL). The mixture was stirred at room temperature for 12 h. The precipitate was filtered off, and the filtrate was dried under vacuum yielding crude product. Pure samples suitable for elemental analysis were prepared by recrystallization from water.
Guanidinium salt 1-c: White solid (94 mg, 80%); mp: 146-148 ℃, Tdec: 258 ℃ (peak, 10 ℃/min); IR (KBr, cm-1): 3508, 3480, 3407, 3344, 3270, 3190, 1554, 1321; 1H NMR (400 MHz, DMSO-d6): δ 6.88 (br s, 1H); 13C NMR (100 MHz, DMSO-d6): δ 176.4, 169.9, 158.4; Elemental analysis calcd. (%) for C3H6N8O5 (234.13): C 15.39, H 2.58, N 47.86, Found: C 15.56, H 2.60, N 47.80.
Aminoguanidinium salt 1-d: White solid (112.1 mg, 90%); mp: 150-152 ℃, Tdec: 205 ℃, 227 ℃ (peak, 10 ℃/min); IR (KBr, cm-1): 3457, 3365, 3174, 1573, 1333; 1H NMR (400 MHz, DMSO-d6): δ 8.55 (br s, 1H), 7.24 (br s, 2H), 6.72 (br s, 2H), 4.68 (br s, 2H); 13C NMR (100 MHz, DMSO-d6): δ 176.4, 169.9, 159.2; Elemental analysis calcd. (%) for C3H7N9O5 (249.14): C 14.46, H 2.83, N 50.60, Found: C 14.56, H 2.91, N 50.47.
Triaminoguanidinium salt 1-e: Yellow solid (118.6 mg, 85%); mp: 147-150 ℃, Tdec: 155 ℃ (peak, 10 ℃/min); IR (KBr, cm-1): 3348, 3318, 3212, 1551, 1389; 1H NMR (400 MHz, DMSO-d6): δ 8.58 (br s, 3H), 4.48 (br s, 6H); 13C NMR (100 MHz, DMSO-d6): δ 176.4, 169.9, 159.5; Elemental analysis calcd. (%) for C3H9N11O5 (279.17): C 12.91, H 3.25, N =.19, Found: C 13.17, H 3.33, N =.25.
3. Results and discussionN-Nitration of NOA with P2O5 and HNO3 gave NON smoothly. Initially, Ba(OH)2 was used to deprotonate its acidic hydrogen, but the barium salt failed to be prepared. To our delight, sodium salt 1-a was formed by addition of solid NaHCO3 to the reaction mixture. Higher yield of 1-a (75%) was obtained with extended reaction time, compared with the literature [10a]. Subsequently, the silver salt 1-b was obtained by treatment of sodium salt 1-a with AgNO3, which is the precursor to prepare 1-c, 1-d and 1-e by metathesis reactions driven by the precipitation of AgCl.
Structures of these energetic saltswere identified by 1H NMR and 13C NMR, IR spectroscopy and elemental analysis. In IR spectra, strong absorptionbands at1562 cm-1 and1335 cm-1 confirmedthe presence of nitro group, while intense absorption bands in the range of 3174 cm-1 to 3457 cm-1 could be assigned to the N-H stretch of the nitrogen-rich cations. In 13CNMR spectra, carbon atoms of 1,2,4- oxadiazole ring were found around 176 ppm and 169 ppm.
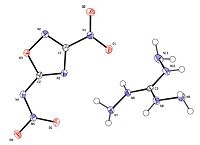
The single crystal of 1-e was obtained from dichloromethaneethanol (5:1, v/v) at roomtemperature. It crystallizes in the triclinic crystal system (P-1) with two molecules in the unit cell. The single crystal density of 1-e is 1.773 g/cm3, locating between 1-c and 1-d (Table 1). In the single crystal structure (Fig. 1), there are three types of hydrogen bonds. The first one occurs between the intramolecular imino group and amino group of cation (N11-H11B...N9 3.5040(16) Å). The second is found between intermolecular amino group of the cation and the oxygen atomof nitro group in the anion (N10-H10...O1 3.0122(13) Å). And the third one takes place between the intermolecular imino group of the cation and the oxygen atom of nitroimino group (N11-H11A…O4 3.0344(12) Å).
|
|
Table 1 Physical properties and calculated detonation performance of 1-c, 1-d and 1-e. |
|
Download:
|
| Fig. 1.Single crystal structure of 1-e. | |
The torsion angle of C(2)-N(4)-N(5)-O(4) is -175.818, and O(1)-N(1)-C(1)-N(2) 174.40°, indicating the nitroimino group and nitro group approximately is coplanar with the 1,2,4-oxadiazole ring. We noted that the torsion angle of N(11)-N(10)-C(3)-N(8) is 180.00°. This fact suggests that the triaminoguanidinium cation is planar (data and parameters of the X-ray measurements and structure refinements are given in Tables S1-S3 in Supporting information. CCDC-1424510 contains supplementary crystallographic data of (1-e). It can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/ data_request/cif.).
The impact sensitivities of the synthesized salts were determined on BAM fall hammer BFH-10 apparatus (5.0 kg drop hammer) with approximately 15 mg samples. As shown in Table 1, the sensitivity of 1-c and 1-d is lower than that of TNT arising from the hydrogen-bonding interactions between the cation and anion. On the other hand, salt 1-e is even more sensitive than RDX [13].
In comparison with NOA, compounds 1-c, 1-d and 1-e have more negative oxygen balance resulting from more amino groups in the nitrogen-rich cations. The thermal stabilities of these energetic compounds were evaluated by DSC at a heating rate of 10 ℃/min. All salts decomposed ranging from 155 ℃ to 258 ℃. Salt 1-c has the highest decomposition temperature at 258 ℃ (Fig. 2). It is shown that as increasing the number of amino group in the guanidinium cation the thermal stability of the resulted energetic salts decreases stepwise [13].

|
Download:
|
| Fig. 2.DSC plot of energetic salt 1-c. | |
Heat of formation of NON anion was calculated using the Gaussian 09 [14] and isodesmic reaction (ΔHfθ 298 K) (Scheme 2).
The geometric optimization of the structures and frequency analyses were accomplished by using the B3LYP with the 6-31+G** basis set [15], and single-point energies were calculated at the MP2/6-311++G** level. Based on the Born-Haber energy cycle (see Eq. (1)), lattice energies were predicted by using the approach of Jenkins et al. [16] (see Eqs. (2) and (3)).
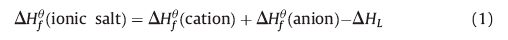


The calculated heats of formation of these energetic salts range from 226 kJ/mol to =4.1 kJ/mol. Therefore, detonation performances were predicted using the Explore 5.0 (6.02 version) program (Table 1). With respect to detonation velocity, the values of 1-c (9319 m/s) and 1-e (9354 m/s) exceed TNT (6881 m/s) and RDX (8997 m/s).
4. ConclusionIn summary, nitrogen-rich energetic salts based on NON were successfully synthesized and fully characterized. The results show that appropriate number of amino or imino group of the cation enhance the thermal stability of the energetic salts as a result of the optimal hydrogen-bonding network. 1-c shows good performance in general. It has low melting points (146-148 ℃) and high decomposition temperature (258 ℃). And the detonation velocity of it exceeds 9300 m/s. It is potential for energetic applications.
AcknowledgmentsWe are grateful of financial support from the National Natural Science Foundation of China (Nos. 21372027 and 21172203). Dr.Xiu-Juan Qi from Institute of Chemical Materials, CAEP is acknowledged for the calculation of detonation velocity and pressure (Explore 5.0 (6.02 version)).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2015.12.008.
| [1] | D.M. Badgujar, M.B. Talawar, S.N. Asthana, P.P. Mahulikar, Advances in science and technology of modern energetic materials:an overview, J. Hazard. Mater. 151(2008) 289-305. |
| [2] | Q.H. Zhang, J.M. Shreeve, Energetic ionic liquids as explosives and propellant fuels:a new journey of ionic liquid chemistry, Chem. Rev. 114(2014) 110527-110574. |
| [3] | M.B. Talawar, R. Sivabalan, T. Mukundan, et al., Environmentally compatible next generation green energetic materials (GEMs), J. Hazard. Mater. 161(2009) 589-607. |
| [4] | P.F. Pagoria, G.S. Lee, A.R. Mitchell, R.D. Schmidt, A review of energetic materials synthesis, Thermochim. Acta 384(2002) 187-204. |
| [5] | J.P. Agrawal, Recent trends in high-energy materials, Prog. Energy Combust. Sci. 24(1998) 1-30. |
| [6] | H.X. Gao, J.M. Shreeve, The many faces of FOX-7:a precursor to high-performance energetic materials, Angew. Chem. (Ⅰ)nt. Ed. 54(2015) 6335-6338. |
| [7] | X. Yin, J.T. Wu, X. Jin, et al., Nitrogen-rich salts of 1-aminotetrazol-5-one:oxygencontaining insensitive energetic materials with high thermal stability, RSC Adv. 5(2015) 60005-60014. |
| [8] | (a) L. Liu, Y.Q. Zhang, Z.M. Li, S.J. Zhang, Nitrogen-rich energetic 4-R-5-nitro-1,2,3-triazolate salts (R=-CH3,-NH2,-N3,-NO2 and-NHNO2) as high performance energetic materials, J. Mater. Chem. A 3(2015) 14768-14778;(b) C.L. He, J.H. Zhang, D.A. Parrish, J.M. Shreeve, 5-Chloro-3,5-dinitropyrazole:a precursor for promising insensitive energetic compounds, J. Mater. Chem. A 1(2013) 2863-2868;(c) Y.C. Li, Q. Cai, S.H. Li, et al., 1,1'-Azobis-1,2,3-triazole:a high-nitrogen compound with stable N8 structure and photochromism, J. Am. Chem. Soc. 132(2010) 12172-12173. |
| [9] | (a) P.M. Jadhav, S. Radhakrishnan, V.D. Ghule, R.K. Pandey, Energetic salts from nitroformate ion, J. Mol. Model. 21(2015) 1-5;(b) H. Wei, C.L. He, J.H. Zhang, J.M. Shreeve, Combination of 1, 2,4-oxadiazole and 1,2,5-oxadiazole moieties for the generation of high performance energetic materials, Angew. Chem. (Ⅰ)nt. Ed. 54(2015) 9367-9371;(c) M.A. Kettner, T.M. Klapçtke, T.G. Witkowski, F. von Hundling, Synthesis, characterisation and crystal structures of two bi-oxadiazole derivatives featuring the trifluoromethyl group, Chem. Eur. J. 21(2015) 4238-4241. |
| [10] | (a) Z.D. Fu, R. Su, Y.Wang, et al., Synthesis and characterization of energetic 3-nitro-1,2,4-oxadiazoles, Chem. Eur. J. 18(2012) 1886-1889;(b) Z.D. Fu, C. He, F.X. Chen, Synthesis and characteristics of a novel, high-nitrogen, heat-resistant, insensitive material (NOG2Tz), J. Mater. Chem. 22(2012) 60-63;(c) Z.D. Fu, Y. Wang, L. Yang, et al., Synthesis and characteristics of novel, high nitrogen 1,2,4-oxadiazoles, RSC Adv. 4(2014) 11859-11861;(d) A.B. Sheremetev, The chemistry of furazans fused to six-and seven-membered heterocycles with one heteroatom, Russ. Chem. Rev. 68(1999) 137-148. |
| [11] | (a) Z.D. Fu, Y. Wang, F.X. Chen, Comparison of thermal performance of new energetic materials NONHT and NONsHT, Acta Armamentarii 34(2012) 235-239;(b) Y. Wang, Z.D. Fu, F.X. Chen, Effect of new energetic materials NOG2TZ to HMX thermal decomposition behavior, Chin. J. Energy Mater. 22(2014) 22-25;(c) Z.D. Fu, Y. Wang, F.X. Chen, The thermal decomposition behavior of new energetic materials NOG, Chin. J. Energy Mater. 20(2012) 583-586. |
| [12] | Y. K. Wu, G. Y. Chen, Z. M. Zhou, H. S. Dong, A method of prediction compound crystal density, CN 101957300 A (2009). |
| [13] | H.X. Gao, J.M. Shreeve, Azole-based energetic salts, Chem. Rev. 111(2011) 7377-7436. |
| [14] | M.J. Frisch, Gaussian 09, Gaussian, (Ⅰ)nc, Wallingford, CT, 2009. |
| [15] | R.G. Parr, W. Yang, Density Functional Theory of Atoms and Molecules, Oxford University Press, New York, NY, 1989. |
| [16] | H.D. Jenkins, D. Tudeal, L. Glasser, Lattice potential energy estimation for complex ionic salts from density measurements, (Ⅰ)norg. Chem. 41(2002) 2364-2367. |
 2016, Vol.27
2016, Vol.27