The chemistry of large lanthanide clusters has long attracted numerous coordination chemists owing to the unique properties conferred on molecules that have f electrons. This is especially true for heterometallic lanthanide/metal multinuclear compounds containing multiple-binding sites, which are unique acceptor systems for alternative interactions with neutral guest molecules and anions. There is a range of applications, such as luminescent sensors [1], catalysts [2, 3, 4], contrast reagents for magnetic resonance imaging [5, 6], and for the preparation of unique magnetic materials [7, 8, 9]. Structure design of such receptors with further controllable integration into frameworks is an amazing possibility. These frameworks may be used as sophisticated materials with a wealth of potential applications.
By the composition of hydroxyl oxime acid and Cu(Ⅱ) atoms forming 15-metallacrown-5 complexes (15-MC-5), the lanthanide embed into the pentagram metallacrown which is researched by Pecoraro and etc. [10, 11, 12, 13], providing a sufficient number of coordination sites located in close proximity to bind substrates. These metallamacro cycles are capable of designing rationally from simple inorganic salts to available organic ligands, such as picoline or isoquinoline hydroxamic acids and related nonplanar amino hydroxamic acids. Such organic ligands may shape two fused fivemembered metalochelate rings, therefore they yield planar pseudo-pentagonal core of 15-MC-5. In this context, the employing of pyrazine parts, via importing pyrazinohydroxamic acid into 15- MC-5 core, offers essential advantages, such as pyridine heterocycle. In this case five additional r-donor groups are introduced into the metallacrowns periphery (Scheme 1). For binding bridging anions, such a ligand modification opens a possibility towards controllable combination of the metallamacrocycle into the framework exploiting its acid amphoteric nature: either donor activity of five exterior nitrogen/oxygen atoms to coordinate bridging metal ions or alternative acceptors properties of the metallacrown metal centers.
|
Download:
|
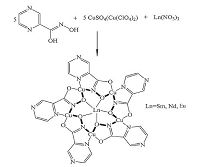
| Scheme 1.The picture presents the way to obtain the copper(Ⅱ)-lanthanide(Ⅲ) 15-metallacrown-5 complexes. | |
In this article, we demonstrate that the fluorescence behavior towards typical solid state structures which are new lanthanide(Ⅲ) encapsulated 15-MC-5 complexes based on pyrazinohydroxamate ligand, are analyzed with inorganic salts (CuSO4, Cu(ClO4)2). The differentiation of atomic and ionic radii between the encapsulated Sm(Ⅲ) can be reasoningly employed as a crucial mood to control bowl-shape distortion of theMCplatform from the planar geometry.
2. ExperimentalThe compound H2Pyzha [14] was prepared according to the literature procedures. All reagents and solvents applied in the syntheses were of reagent grade and used without further purification. Infrared spectra were recorded on a Nicolet AVATAT FT-IR360 spectrometer as KBr pellets in the frequency range 4000- 400 cm-1. Photoluminescence measurements were performed on a Hitachi F-4500 fluorescence spectrophotometer with solid powder on a 1 cm quartz round plate.
2.1. Preparation of the metallacrownsIn a typical procedure, 10 mL solution of CuSO4-5H2O (124.8 mg, 0.5 mmol) and Sm(NO3)3-6H2O (44.5 mg, 0.100 mmol) was mixed with 5 mL solution of H2Pyzha (69.6 mg, 0.500 mmol) in MeOH. It immediately caused a change in color from blue to concentrated dark green. The relevant solution was stirred at room temperature for 24 h followed by evaporation to yield compound 1. IR (KBr, cm-1): 3460 (s), 2891 (m), 1703 (s), 1564 (s), 1330 (m), 1247 (m), 1068 (m), 852 (w), 690 (w), 576 (w), 461 (m). The syntheses of 2,3 were essentially the same as compound 1. Using the same molar mass of CuClO4-6H2O to replace CuSO4-5H2O and Nd(NO3)3-6H2O, with Eu(NO3)3-6H2O in place of Sm(NO3)3-6H2O, yields compound 2 and 3. Compound 2: IR (KBr, cm): 3271 (s), 2991 (m), 2191 (m), 1883 (s), 1591 (s), 1337 (m), 1221 (m), 1001 (m), 845 (w), 699 (w), 547 (w). 3: IR (KBr, cm-1): 3312 (s), 27= (m), 2103 (m), 1773 (s), 1608 (s), 1285 (m), 1147 (m), 998 (m), 872 (w), 688 (w), =2 (w), 433 (m), 237 (m).
2.2. X-ray crystallographyThe crystallographic measurements for the complexes were carried out with an Oxford-Diffraction diffractometer (XCALIBUR E CCD) equipped with graphite-monochromated Mo Kα radiation. A single crystal was positioned at 45 mm from the detector and 311 and 383 frames were measured each for 50 and 75 s over 1 scan width for the metallacrown. The unit cell determination and data integration was done using the CrysAlis package of Oxford Diffraction. All absorption corrections were performed using the SADABS program [15]. All the structures were solved by direct methods using Olex2 [16] software with the SHELXS structure solution program and refined by full-matrix least-squares on F2 with SHELXL-97 [17]. All the non-hydrogen atoms were treated anisotropically. The positions of hydrogen atoms were generated geometrically. The crystallographic details are summarized in Table S1 and selected bond lengths are displayed in Table S2, which are present in Supporting information.
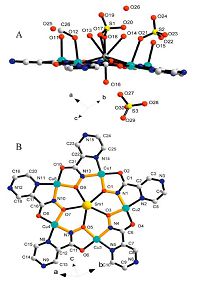
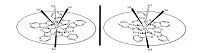
3. Results and discussion 3.1. Crystal structure 1In the solid state these features have been confirmed by X-ray analysis. The crystal structure of molecular compound [Sm(H2O)3 {Cu(pyzha)}5 (H2O)2 (MeOH) (HSO4)2]·(H2O)2·(HSO4) reveals a racemic mixture of two enantiomers consisting of a typical metallacrown core (Fig. 1 and Table S2). As previously shown [18], two faces of the 15-MC-5 pentagonal plane could be identified by the distribution of molecules axially bounded to rare earth element: Sm(Ⅲ). In the case of the 15-MC-5 pyrazinohydroxamates, this causes chirality within the molecular macrocyclic structure, generating a pair of enantiomers: clockwise (C) with the cyclic Cu-N-O orientation and anticlockwise (A) with the opposite Cu-N-O orientation (Scheme 2). Either of the five pyrazinohydroxamic ligands is double deprotonated and forms two mixed five-membered metalochelates with the two ring-forming Cu(Ⅱ) atoms.
|
Download:
|
| Fig. 1.The Molecular structure of Sm(Ⅲ) encapsulated 15-MC-5 complexes has been presented: (A) side view of complex; (B) top view of [Sm (H2O)3 {Cu(pyzha)}5 (H2O)2 (MeOH) (HSO4)2]·(H2O)2·(HSO4) (a ring based on the -Cu-N-O-unit is outlined in bold orange line). | |
|
Download:
|
| Scheme 2.The picture shows chirality within the molecular macrocyclic structure generating a pair of enantiomers: clockwise (C) with the cyclic Cu-N-O orientation and anticlockwise (A) with the opposite Cu-N-O orientation. | |
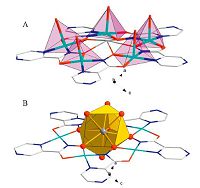
For the complex, the cavity radius of the macrocycle is about 1.12 Å , and the ionic radius of the nine-coordinated samarium ion (1.13 Å [19]) (Fig. 2A) provides the perfect location for the lanthanide in the center of the metallacrown with negligible displacement from the least-square plane of five hydroxamate oxygen atoms (±0.02 Å). The coordination sphere of the Sm(Ⅲ) ion is completed by five equatorial oxygen atoms of hydroxamates (The Sm-O is about 2.49-2.55 Å) and three axial H2O groups and a HSO4- group, which are symmetrically combined with the metal center above and below the MC platform (Sm-O is about 2.45-2.69 Å) and the distances of the bonds of the Sm-O are all the same [20]. The coordinated HSO4-, H2O, and MeOH ions occupy the apical positions of the square-pyramidal Cu(Ⅱ) atoms from one side of the metallacrown, forming a complete coordination shell (Fig. 2B) with normal bond length [21]. Because the metallacrown face-discrimination phenomenon towards the integrated solvent molecules or ions results in a non-planar distortion of the 15-MC-5 core, a metallamacrocycle assumes a cone-shaped geometry. The deviation from planarity is evidenced by displacements of the cavity center of the metallacrown from the least-square plane of the five peripheral pyrazine nitrogens. Presumably, such a distortion effect is dependent upon the number of coordinated molecules, their molecular size, and their distribution on either metallacrown platform. Interestingly, in the coordination structure, the sulfurous acid groups demonstrate different binding modes: one HSO4- group is a bridging-bidentate mode between the lanthanide atom and the Cu(Ⅱ) atom, while the other group has two roles: chelating only with Cu(Ⅱ) atom and a detached mode in the coordination structures, which all obtain H+ from the ligands.
|
Download:
|
| Fig. 2.The picture reveals complex geometric structure: (A) the six-coordinated of Cu(Ⅱ) ions; (B) the nine-coordinated of Sm(Ⅲ) atom. | |
The crystal packing behavior of the metallacrown, possessing a distinct bowl-shape geometry and large surface area (~221 Å2), offers the opportunity to form intermolecular cavities which are perfectly suitable for accommodating unbound solvent guests (e.g., HSO4-, H2O) (Fig. 3). Tight packing of the metallamacrocycles is also obtained through intermolecular face-to-face (d = 3.431 Å) or offset face-to-face π-π stacking interaction (z = 3.983 Å , d = 3.466 Å) [22] (Fig. 3).
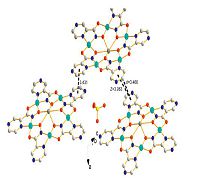
|
Download:
|
| Fig. 3.The picture reveals the crystal packing view of molecular metallacrown illustrating the accommodation face-to-face or offset face-to-face π-π stacking interaction. | |
The X-ray crystal structure analysis reveals that complex 2 and 3 crystallize in the monoclinic space group Cc. Their multiplebinding pentagonal platforms are the same with compound 1 while the side view of the structure is very difference, which is shown in Fig. 4A. Because there are no enantiomers in the structures, they have non centro-symmetric space group. The coordinate Cu atoms (Fig. 4B) have two modes to combine, one of which adopts a distorted octahedral geometry, coordinating by two free molecules (orange in the picture), two nitrogen atoms of ligand, and two oxygen atoms of deprotonated pyrazinohydroxamic acid. However the five coordinated Cu atoms (two of five) have tetragonal-pyramidal geometry (pink in the picture) whose tops are located on different sides of the main platform. When it comes to rare earth elements, they have eight coordination, forming a dodecahedron structure (red in the picture). The eight-coordinated environment of Ln(Ⅲ) is completed in the axial positions by methyl alcohol molecules (Nd, Eu-O 2.36, 2.44 Å), on the opposite side, two of which occupy the convex face of the metallacrowns. The distances between Cu and O are longer than the normal one (The longest is about 2.8 Å). Because of the John-Teller effect, six coordinated Cu atoms always form elongated distorted octahedrons. As shown in Fig. 5 (pink dotted lines), the coordinated anions and aqua molecules form complicated intermolecular hydrogen bonding interactions. The first formation of a hydrogen bonded with another two metallacrowns. These hydrogen bonds and angles (O(18)…O(20) = 2.837(3) Å , O(20)- H(20B)…O(18) = 157.24(2)°; N(3)…O(22) = 2.845(1) Å , O(22)- H(22)…N(3) = 160.39(2)°, B = -1/2 + x, 1/2 + y, z for complex 2; O(18)…O(20) = 3.=5(7) Å , O(20)-H(20B)…O(18) = 154.66(2)°; N(3)…O(22) = 2.884(1) Å , O(22)-H(22)…N(3) = 165.94(4)°, B = x, -y, 1/2 + z.) for complex 3, are all similar. In the crystal lattice, the intermolecular hydrogen bonding interactions define the integration of the metallacrown dimers into the 3-D networks.

|
Download:
|
| Fig. 4.This is the molecular structure of two Ln(Ⅲ) encapsulated 15-MC-5 complexes (Ln=Nd3+, Eu3+): (A) side view of complex; (B) description of complex geometric structure. | |
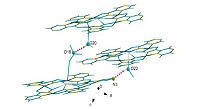
|
Download:
|
| Fig. 5.The picture illustrates the hydrogen bonds between molecular metallacrowns. | |
Crystallographic data for the structures of 1-3 in the form of CIF files have been deposited with the Cambridge Crystallographic Data Center as supplementary publication Nos. 1427967, 1432230, 1432231 for the crystals, respectively. Copy of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 IEZ, UK (fax: +44 1223 336 033; e mail: deposit@ ccdc cam ac uk).
3.3. Fluorescence propertiesLanthanide ions are widespread employed in constructing metal-organic frameworks (MOFs) for their potential in photonic and magnetic applications. In the past decade, the exploration of heterometal-organic frameworks containing lanthanide metal and d10 metal complexes has attracted much attention for their fascinating topologies with interesting luminescence properties [23]. The solid-state luminescence of the compound was investigated at room temperature (Fig. 6). Upon excitation at 381 nm, the compound displays the characteristic emission of Sm(Ⅲ). The three characteristic peaks at 435, 474, and 536 nm are attributed to Sm(Ⅲ) transitions from 4G5/2 to 6FJ (J = 5/2, 7/2, 9/2), respectively. The most intense peak is the transition 4G5/2→6F7/2 at 474 nm The luminescence investigations on the complex mentioned above suggested that the ligand may effectively sensitize the luminescence of Sm(Ⅲ) ions. Compounds 2 and 3 are similar to each other and demonstrate different fluorescence from compound 1. They reflect the fluorescence of Cu atoms which buried the fluorescence properties of the lanthanide ions. The transitions of f*-f is a forbidden transition, while by introducing appropriate organic ligands and rare earth ions, the luminescence of the central ions can be effectively enhanced in the molecules, which can enhance the fluorescence properties of rare earth ions. This phenomenon is called the "Antenna" effect of the ligands. Although there are electronic ions in Nd3+ and Eu3+, there are great ranges of the electric transition energy levels, and the energy level spacing between spectral terms of the ground state and excited states are small. When the lanthanide is excited by the triplet energy ligands, electronic transitions between the spectral terms will produce strong radiation inactivation, so the fluorescence of these two kinds of rare earth ions are usually weak. Sometimes they reflect the fluorescence of transition metals, especially Cu atoms. When it comes to Sm(Ⅲ), the non-radiation loss of f*-f transition is probably small, therefore the Sm(Ⅲ).
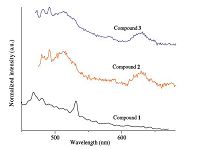
|
Download:
|
| Fig. 6.The solid-state fluorescence spectra of the three metallacrowns are depicted (λex = 381 nm). | |
We have introduced three Cu(Ⅱ)-lanthanide(Ⅲ) 15-metallacrown- 5 complexes based on pyrazinohydroxamic acid as a new multiple-binding platform bearing acid amphoteric properties. The complex 1 has discrete HSO4- ions in 15-MC-5 core, which could be three types: one is a bridging-bidentate between the Sm(Ⅲ) ion and the Cu(Ⅱ) cation; another part chelates only with the Cu(Ⅱ) atom; and the remaining part is a free mode in the solid structure. Reaction of molecular metallacrowns with excess CuSO4 proceeds as an anion metathesis process affording heteroanionic metallacrowns. Another two structures can form 3-D structures through hydrogen bonds. For complex 1, the ligands cause stronger fluorescence of the lanthanides than in the other complexes. Sm(Ⅲ) is the only lanthanide in the three crystals that cannot be affected by Cu atoms in this kind of ligands.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2015.12.006.
| [1] | Z.L. Wu, J. Dong, W.Y. Ni, et al., Unique chiral interpenetrating d-f heterometallic MOFs as luminescent sensors, (Ⅰ)norg. Chem. 54(2015) 5266-5272. |
| [2] | H.C. Aspinall, Chiral lanthanide complexes:coordination chemistry and applications, Chem. Rev. 102(2002) 1807-1850. |
| [3] | Q.B. Yuan, S.L. Zhou, X.C. Zhu, et al., Heterometallic rare-earth metal complexes with imino-functionalized 8-hydroxyquinolyl ligands:synthesis, characterization and catalytic activity towards hydrophosphinylation of trans-β-nitroalkene, New J. Chem. 39(2015) 7626-7632. |
| [4] | J.J. (Ⅰ)nanaga, H. Furuno, T. Hayano, Asymmetric catalysis and amplification with chiral lanthanide complexes, Chem. Rev. 102(2002) 2211-2226. |
| [5] | A.J. Stemmler, J.W. Kampf, M.L. Kirk, et al., The preparation, characterization, and magnetism of copper 15-Metallacrown-5 lanthanide complexes, (Ⅰ)norg. Chem. 38(1999) 2807-2817. |
| [6] | A. Svitova, K. Braun, A.A. Popov, et al., A platform for specific delivery of lanthanide-scandium mixed-metal cluster fullerenes into target cells, Chem. Open 1(2012) 207-210. |
| [7] | C.M. Zaleski, E.C. Depperman, J.W. Kampf, et al., Synthesis, structure, and magnetic properties of a large lanthanide-transition-metal single-molecule magnet, Angew. Chem. (Ⅰ)nt. Ed. 43(2004) 3912-3914. |
| [8] | Y.F. Zhou, F.L. Jiang, D.Q. Yuan, et al., Copper complex cation templated gadolinium(Ⅲ)-isophthalate frameworks, Angew. Chem. (Ⅰ)nt. Ed. 43(2004) 5665-5668. |
| [9] | J.J. Zhang, S.M. Hu, S.C. Xiang, et al., Syntheses, structures, and properties of highnuclear 3d-4f clusters with amino acid as ligand:{Gd6Cu24} {Tb6Cu26}, and {(Ln6Cu24)2Cu} (Ln=Sm, Gd), (Ⅰ)norg. Chem. 45(2006) 7173-7181. |
| [10] | A.D. Cutland, R.G. Malkani, J.W. Kampf, V.L. Pecoraro, Lanthanide 15-metalla-crown-5 complexes form nitrate-selective chiral cavities, Angew. Chem. (Ⅰ)nt. Ed. 39(2000) 2689-2692. |
| [11] | T.N. Parac-Vogt, A. Pacco, P. Nockemann, et al., Relaxometric study of copper 15-metallacrown-5 gadolinium complexes derived from α-aminohydroxamic acids, Chem. Eur. J. 12(2005) 204-210. |
| [12] | C.S. Lim, J. Jankolovits, P. Zhao, et al., Gd(Ⅲ)[15-metallacrown-5] recognition of chiral alpha-amino acid analogues, (Ⅰ)norg. Chem. 50(2011) 4832-4841. |
| [13] | G. Mezei, C.M. Zaleski, V.L. Pecoraro, Structural and functional evolution of metallacrowns, Chem. Rev. 107(2007) 4933-5033. |
| [14] | B.S. Kushner, H. Dalalian, J.L. Sanjurjo, et al., Experimental chemotherapy of tuberculosis(Ⅱ). The synthesis of pyrazinamides and related compounds, J. Am. Chem. Soc. 74(1952) 3617-3621. |
| [15] | G.M. Sheldrick, SADABS:Program for Empirical Absorption Correction of Area Detector Data, University of Göttingen, Göttingen, Germany, 1996. |
| [16] | O.V. Dolomanov, L.J. Bourhis, R.J. Gildea, J.A.K. Howard, H. Puschmann, OLEX2:a complete structur solution, refinement and analysis program, J. Appl. Crystallogr. 42(2009) 339. |
| [17] | G.M. Sheldrick, SHELXS-97, Structure Solving Program and Program for the Refinement of Crystal Structures from Diffraction Data, University of Göttingen:Göttingen, Germany, 1997. |
| [18] | V.L. Pecoraro, J.J. Bodwin, A.D. Cutland, Formation of chiral solids via a molecular building block approach, J. Solid State Chem. 152(2000) 68-77. |
| [19] | E. Kusrini, M.(Ⅰ). Saleh, R. Adnan, et al., Ternary complexes of neodymium(Ⅲ) and samarium(Ⅲ) picrate triethylene glycol:structural, spectroscopic, and photoluminescent properties, J. (Ⅰ)nclusion Phenom. Macrocyclic Chem. 74(2012) 425-436. |
| [20] | J.J. Zhang, T.L. Sheng, S.Q. Xia, et al., Syntheses and characterizations of a series of novel Ln6Cu24 clusters with amino acids as ligands, (Ⅰ)norg. Chem. 43(2004) 5472-5478. |
| [21] | P.M. Guha, H. Phan, J.S. Kinyon, et al., Structurally diverse copper(Ⅱ) complexes of polyaza ligands containing 1,2,3-triazoles:site selectivity and magnetic properties, (Ⅰ)norg. Chem. 51(2012) 3465-3477. |
| [22] | C. Janiak, A critical account on π-π stacking in metal complexes with aromatic nitrogen-containing ligands, J. Chem. Soc., Dalton Trans.21(2000) 3885-3896. |
| [23] | M. Sakamoto, K. Manseki, H. Okawa, D-f heteronuclear complexes:synthesis, structures and physicochemical aspects, Coord. Chem. Rev. 381(2001) 379-414. |
 2016, Vol.27
2016, Vol.27 


