Hydrogen-bonded water clusters or networks have attracted significant attention because water clusters can act as promising models for the study of water and water clusters in biological, chemical, and physical processes [1, 2, 3, 4]. Growing larger clusters and controlling the linkage mode of small clusters to form large networks, which is the bridge between water clusters and bulk water, is still a challenging scientific endeavour [5, 6].
Anion-water aggregation is an important branch of water chemistry. The negative charges and rich binding sites on anions greatly facilitate the formation of hydrogen bonds among water molecules. The water clusters with the participation of anions have larger numbers of water molecules and more interesting spatial structures, which is important in understanding the behaviour of anions in water-based biological systems [7, 8]. According to the literature, extensive research efforts have been devoted to inorganic anion-water aggregation [9, 10, 11, 12, 13, 14, 15]. However, organic anion-water aggregates and their influences to the formation of water clusters have rarely been explored.
Water clusters need certain frames to support their structures, including coordination compounds and organic compounds [16, 17]. Compared to metal-organic complexes, organic frames have their own advantages in simulating biological systems. The stability of the water clusters contributes significantly to the investigation of biological molecules and stereochemistry. These networks also play an important role in the self-assembly process involving an intricate array of hydrogen-bonding interactions in the organism, which could insert, exchange and co-constitute the structures with biomolecules [18, 19]. However, water clusters have not been extensively studied in a wide range of organic compounds systems, in particular, for supramolecular assembly systems.
Cucurbit[6]uril (CB[6]) is a unique macrocyclic host molecule, in which the hydrophobic cavity can provide a potential inclusion site for nonpolar units such as aliphatic chains and aromatic rings [20, 21]. The polar carbonyl groups at the portals allow CB[6] to bind cations and charged molecules through charge-dipole and hydrogen-bonding interactions [22, 23]. CB[6] is often utilized for the construction of supramolecular architectures, such as (pseudo)rotaxanes, polyrotaxanes and molecular necklaces [24, 25, 26, 27, 28, 29]. In our previous studies, the construction of [Br(H2O)3]- [30] and [(COO)2(H2O)10]2- [31] in CB supramolecular systems were successfully constructed. In this study, the CB[6] supramolecular system was selected as frames to support water clusters. Therefore, consider that organic carboxylate anionic species with abundant hydrogen bonding sites may offer suitable hydrophilic environments beneficial for the existence of water clusters, organic carboxylate anions were added as templates to study the factors of spatial structures of water clusters.
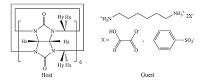
Herein, we report the crystal structure of two supramolecular architectures using CB[6] as the host and hexamethylene diamine salt as the guest (Fig. 1), namely {(HC2O4)2 2-[C6H18N2 2+ ⊂ C36H36- C36H36N24O12]}·12H2O (1) and {(C6H5SO3)2 2-[C6H18N2 2+ ⊂ C36H36- C36H36N24O12]}·12H2O (2), which involved hydrogen oxalate and benzenesulfonate as anionic templates. Two dimensional infinite L18(8)14(8)8(4) type anion-water aggregates [(HC2O4)4 (H2O)22]4- and one dimensional "W"-like T5(0)A2 type anion- water clusters [(C6H5SO3)(H2O)6]- were encapsulated in these two supramolecular compounds. Hexamethylene diamine salt was used in each supramolecular compound in order to avoid the interruption caused by different guests in the structures. The two new anion-water clusters can help to delineate the effect of the space structure of anions and the positions of oxygen atoms on water clusters in CB’s supramolecular systems. The effect of intermolecular interactions on the architectures was also analyzed by Hirshfeld surface analyses. The stability and the process of dehydration and rehydration of water clusters were profiled by thermogravimetric analysis (TGA) curves and X-ray powder diffraction (XRD).
|
Download:
|
| Fig. 1.The structure of host cucurbit[6]uril and the guests hexamethylene diamine salts. | |
CB[6] was prepared according to the literatures [18, 20]. A mixture of CB[6] (1.20 g, 1.20 mmol) and 1, 6-hexyl-di(ammonium hydrogen oxalate) (0.296 g, 1.0 mmol) in 50 mL water was heated at 105 ℃ for 12 h. After stirring at room temperature overnight, the mixture was filtered and heated at 80 ℃ in the reactor for 1 day. X-ray quality white crystals of pseudorotaxane 1 were obtained after slowly cooled to room temperature with the yield of 73%. 1H NMR (400 MHz, D2O): δ 0.453-0.524 (m, 4H, H1), 0.650 (s, 4H, H2), 2.740-2.764 (m, 4H, H3), 4.325-4.354 (d, 12H, J = 11.6 Hz, Hx), 5.617 (s, 12H, Hz), 5.760-5.779 (d, 12H, J = 7.6 Hz, Hy); IR (KBr, cm-1): 3003, 2362, 1736, 1624, 1471; ESI-MS: m/z: 1113.3 [C36H36N24O12 + C6H18N2-H]+.
The synthesis of pseudorotaxane 2 is similar to 1. A mixture of CB[6] (1.20 g, 1.20 mmol) and 1, 6-hexyl-di(ammonium benzenesulfonate) (0.434 g, 1.0 mmol) in water (50 mL) was heated at 110 ℃ for 24 h. After stirring at room temperature overnight, the residue was filtered off. The mixture was heated at 80 ℃ in the reactor for 1 day and then slowly cooled to room temperature to get X-ray quality white crystals of pseudorotaxane 2 with the yield of 65%. 1H NMR (400 MHz, D2O): δ 0.48-0.52 (m, 4H, H1), 0.65 (m, 4H, H2), 2.92-2.95 (t, 4H, H3), 4.35-4.39 (d, 12H, J2 = 7.6 Hz, Hx), 5.61 (s, 12H, Hz), 5.71-5.74 (d, 12H, Hy), 7.80 (m, 2H, Ha), 7.53- 7.62 (m, 3H, Hb, Hc); IR (KBr, cm-1): 2927, 1732, 1473; ESI-MS: m/z: 1272.2 [C36H36N24O12 + C6H5SO3+ C6H18N2]+.
2.2. Single crystal X-ray crystallographySingle-crystal X-ray diffraction data were measured on a Bruker SMART APEX Ⅱ CCD diffractometer with Mo Kα radiation (λ = 0.71073 Å ) at 173(2) K. The structures were solved by the direct method using the (SHELXL-97) and refined against F2 in anisotropic approximation (SHELXL-97). The crystallographic data, data collection conditions and refinement parameters for compounds 1 and 2 are listed in Table 1.
|
|
Table 1 Crystal structures, data collections and structure refinement parameters for pseudorotaxane 1 and 2. |
The single crystal X-ray analysis of pseudorotaxane 1 reveals the guest molecule hexamethylene diamine cation is threaded into the cavity of CB[6] by intermolecular interactions and two HC2O4- anions serve as counter ions to balance the charge. In the crystal structure, the supramolecular architecture contains 3D sandwichlike networks constructed by alternating C6H18N2 ⊂ C36H36N24O12 units and anion-water clusters (Fig. 2).

|
Download:
|
| Fig. 2.The detail crystal structures of pseudorotaxane 1. (a) Hydrogen bonding motif 2D-layered anion water aggregates [(HC2O4)4(H2O)22]4-, (b) (H2O)6 water clusters, (c) hydrogen bonds of the host-water interactions and (d) sandwich-like supramolecular networks along the c axis. (From the guest molecule, some hydrogen bonds are omitted for clarity.). | |
Sandwich-like supramolecular networks exhibit remarkable features involving 2D infinite L18(8)14(8)8(4) type anion-water aggregates [(HC2O4)4(H2O)22]4- in compound 1 along the boc layer. HC2O4- acts as a anionic template and induces six independent water molecules into the positions favourable for the formation of a 2D H2O-HC2O4- anion layer {[(HC2O4)4(H2O)22]4-}n by hydrogen bonds (Fig. 2a and b). Interestingly, the complicated water layers contain large holes defined by 12 water molecules. The HC2O4- anions were located at the edges of the circular holes and act as bridges to link different (H2O)6 water clusters through hydrogen bonds between the individual (H2O)6 water clusters and the oxalate groups. Each HC2O4- anion supplies three O atoms (O7, O9, and O10) and binds four lattice water molecules (O14W, O11W, O12W, and O14'W) through four hydrogen bonds (O—O: in the range of 2.561-2.992 Å ). The O…O distance in the (H2O)6 water cluster is in the range of 2.655-2.852 Å with an average value of 2.745 Å, which is extremely close to the distance of 2.74 Å in ice Ic [32], and 2.76 Å in ice Ih [33].
The 2D layer of anion-water aggregates consists of one 18-, one 14- membered macrocycles and two 8-membered small rings as loops. The 18-membered ring consists of two HC2O4- anions and ten water molecules while the 14-membered ring consists of two HC2O4- anions and eight water molecules. The 18-membered ring shares four water molecules (O11W, O14W, O15W, and O16W) with the 14-membered ring as the shared edge. Both rings are arranged alternately in a ladder-like conformation along the c axis. 8-membered rings are connected with each other through HC2O4- anions to form a necklace-like structure. Ladder-like and necklacelike conformations share common edges comprising HC2O4- anion and two water molecules (O11W, and O12W). The 2D anion-water layers are stacked one by one in parallel along a axis.
Pseudorotaxane C6H18N2 2+ ⊂ C36H36N24O12 act as pillars and connect the 2D anion-water layers through eight hydrogen bonds (Fig. 2c). One CB[6] is connected to four lattice water molecules (O12W, O13W, O15W, and O16W) present in one anion-water layer, and four lattice water molecules (O12'W, O13'W, O15'W, and O16'W) in another anion-water layer. Pseudorotaxanes C6H18N22+ ⊂ C36H36N24O12 are stacked in tubular style with the distance of repeating unit of 12.746 Å and inserted into the anion- water layers along c axis. The average distance between two neighbouring supramolecular tubes at the same layer is 10.081 Å and the distance between the consecutive tubes layer is 13.160 Å. Alternating anion-water layer and tube layer form 3D sandwichlike supramolecular networks (Fig. 2d).
The crystal analysis of 2 reveals that each unit cell contains four asymmetric units {(C6H5SO3)2 2-[C6H18N22+ ⊂ C36H36- C36H36N24O12]}·12H2O. Similar to compound 1, compound 2 also contains C6H18N22+ ⊂ C36H36N24O12 as frames to support water clusters and benzenesulfonate is used as a counter anion. Compared to compound 1, the cell parameters of compound 2 vary significantly, which exhibits bigger size than compound 1 and the crystallographic system changes from monoclinic to orthorhombic crystal lattice. The results may be explained by the difference of two kinds of counter anions.
Supramolecular compound 2 contains honeycomβ-like 3D networks. The remarkable feature of the network is 1D "W"-like T5(0)A2 type anion-water clusters [(C6H5SO3)(H2O)6]- (Fig. 3a). In the structure, C6H5SO3- acts as an anionic template and induces six independent water molecules into the positions that are favourable for the formation of hydrogen bond-driven water-C6H5SO3- anion clusters (Fig. 3b). All the units of anion clusters [(C6H5SO3)(H2O)6]-} are interconnected through the hydrogen bonds (O12-H12...O9) between the O9 atoms of C6H5SO3- anions and water O12W atoms. C6H5SO3- anion uses the remaining O atoms (O7, and O8) to link the water molecules (O14W, O15W) of the (H2O)6 water clusters. The structure of (H2O)6 water cluster is branch shaped, which is different from that in compound 1. The O…O distance in the (H2O)6 water cluster is in the range of 2.750- 3.125 Å with an average value of 2.882 Å, which is close to the value of 2.85 Å in liquid water [31]. Compared to compound 2, it is significantly larger.

|
Download:
|
| Fig. 3.The detail crystal structure of pseudorotaxane 2 (a) hydrogen bonding motif "W"-like T5(0)A2 tape anion water clusters [(C6H5SO3)(H2O)6]-, (b) (H2O)6 water cluster, (c) hydrogen bonds of the host-water interactions and (d) honeycomb-like supramolecular networks along the b axis. (Form the guest molecules, some hydrogen bonds are omitted for clarity.). | |
The W-like [(C6H5SO3)(H2O)6]- water cluster chains at opposite position can form honeycomb-like 2D anion-water cluster layer along aoc square. Different layers stack one by one in a parallel fashion along b axis with a distance of 10.033 Å between two neighbouring layers. However, the pores of the channel are not vertical for the distortion of the stack. Pseudorotaxane C6H18N22+ ⊂ C36H36N24O12 is inserted into the pores and bind anion-water clusters through ten hydrogen bonds (Fig. 3c). Each CB[6] uses its polar carbonyl groups (O1, O2, O3, O5, and O6) to connect water molecules (O11W, O12W, O13W, O14W, O16W). The O…O distance of water-CB[6] spans the range between 2.762- 2.916 Å with an average value of 2.819 Å . The pseudorotaxane can form honeycomb-like structure extending along a axis with a distance of 12.469 Å in each repeating unit and these units constitute 3D networks (Fig. 3d).
The CIF files of the crystal structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre (CCDC) with the Nos. 1024927 and 1024928 for 1 and 2, respectively. The data can be obtained free of charge from CCDC via www.ccdc.cam.ac.uk/data_request/cif.
3.2. Structural influence of anion templates on water clusterNormally, the structures of water clusters are constructed both by the supramolecular systems and the anion templates. In these two compounds, we choose same host-guest systems to avoid the interruption caused by different guests in the structures. Compared the crystal structures, we can find that the structural diversities of water clusters are modulated by two different anions, hydrogen oxalate and benzenesulfonate, which seems to be affected by the spatial structure of anions. The spatial structure of hydrogen oxalate is planar and symmetrical with the point group D2h. In an oxalate molecule, four O atoms are located at two sides of the C-C skeleton with an angle of approximately 180° and stretch to four directions in-plane, which could act as the acceptors in hydrogen bonds. In compound 1, only three O atoms in one oxalate anion serve as binding sites to connect with (H2O)6 water clusters for the directivity and saturability of hydrogen bond and cause the formation of 2D planar-like anion-water aggregates, which have a tight and highly ordered structure. While benzenesulfonate has an asymmetrical structure with the point group C3v, in which three O atoms of the sulfonate locate on one side of the phenyl group and stretch to three directions. There are two spatial orientation of benzenesulfonate and all binding sites are located at the same side of the molecule in the crystal structure. When the O atoms of benzenesulfonate anions bind with (H2O)6 and form anion-water aggregates, the water cluster structures distort and shape 1D W-like, which are much looser and have lower symmetry than oxalate anions (Fig. 4). The results present a kind of theoretical model of water clusters: planar organic anion templates with symmetrical binding sites tend to modulate water clusters to form 2D structures in supramolecuar systems, while low symmetric organic template might lead to less symmetrical water cluster aggregates in solid. Such phenomena were also observed in those benzene dicarboxylic acids or tricarboxylic acids anion participated metal organic frame systems [14, 15]. These results indicate that we may choose organic anion templates with certain structures to adjust the spatial structures of water cluster aggregates in organic supramolecular systems and simulate the participation of organic anion clusters during the self-assembly of biosystems.

|
Download:
|
| Fig. 4.Possible imaged hydration models of oxalate and benzenesulfonate anions (green areas). | |
The Hirshfeld surface and fingerprint plots were utilized for visualizing and analyzing the intermolecular interactions in crystals because they can identify the types and regions of intermolecular interactions based on the electronic distribution calculated as the sum of spherical atom electronic densities [34, 35], which were generated using the Crystal Explorer 3.1 software [36]. The normalized contact distance (dnorm) based on both de (de distance from the surface to the nearest atom exterior to the surface), di (di distance from the surface to the nearest atom interior to the surface) and the van der Waals radii of the atom is given by Eq. (1). The combination of de and di in the form of a 2D fingerprint plot provides a summary of intermolecular interactions in the crystal.

The Hirshfeld surface of compounds 1 and 2 was mapped over dnorm. The information listed in the hydrogen bonding Tables S1- S4 was summarized effectively in these spots, with the large circular depressions (red) visible on the surface and indicative of hydrogen bonding interactions (Fig. 5). The intermolecular interactions between the carbonyl groups in the host molecule and oxygen atoms in the water molecules can be clearly seen in the Hirshfeld surface as the bright red areas in Fig. 6 due to the C=O…H (carbonyl) and O-H…O (water) interactions. The light red spots are due to C-H…O interactions, and other visible spots on the surface correspond to the H…H interactions. The O…H/ H…O intermolecular interactions appear as distinct spikes in the 2D fingerprint plot.

|
Download:
|
| Fig. 5. Hirshfeld surfaces mapped with dnorm (a) pseudorotaxane 1 and (b) pseudorotaxane 2. The surfaces are shown as transparent to allow visualization of the orientation and conformation of the functional groups in the molecules. | |
|
Download:
|
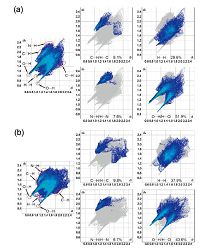
| Fig. 6.Fingerprint plots of the (a) pseudorotaxane 1 and (b) pseudorotaxane 2: completely into C…H, H…H, N…H and O…H contacts showing the percentages of interactions contributed to the total Hirshfeld surface area of the molecule. | |
Complementary regions are visible in the fingerprint plots where one molecule acts as a donor (de > di) and the other as an acceptor (de < di). The fingerprint plots could be divided in order to highlight particular close interactions between two atoms [35]. This decomposition enables the separation of contributions from different types of interactions in the entire fingerprint region. In the fingerprint plots of compound 1 and 2, there are two sharp spikes pointing toward the lower left of the plots and are attributed to typical O-H…O hydrogen bonds, which all belong to anion- water aggregates (Fig. 6). The shortest contact i.e., the minimum value of (de + di) is ~1.75 Å, which signifies the importance of these interactions. The proportions of O…H/H…O interactions contribute 51.9% and 43.6% of the total Hirshfeld surface for compound 1 and 2, respectively. Such difference may be caused by the density of water aggregates in crystals. Compared compound 1 and 2, the distribution of [(HC2O4)4(H2O)22]4- anion-water clusters in crystal is tighter than that of [(C6H5SO3)(H2O)6]-. A more significant contribution is H…Hinteractions (29.6%, 37.9% in compound 1 and 2) derived from the considerable engagement of hydrophobic groups in the self-assembly of molecular framework. According to these results, the proportions O…H and H…H interactions are the largest part in all interactions both in compounds 1 and 2. These indicate that hydrogen bonding and hydrophobic interactions are the dominate drive forces in the formation of supramolecular system, which are consistent with the results of the single-crystal structure analysis.
3.4. Thermogravimetric analysis (TGA), dehydration and rehydration studiesThermal stability of the synthesized complexes was investigated by TGA. The TGA curves show that the mass loss of pseudorotaxanes 1 and 2 occur in three steps (Fig. S3 in Supporting information). Compound 1 undergoes dehydration below 104 ℃ corresponding to the loss of water molecules (calcd. 14.3%, absd. 13.8%). The structure is thermally stable up to 225 ℃ followed by the decomposition of the anhydrous compound and the weight loss in the temperature range 225-470 ℃. Compound 2 undergoes dehydration below 100 ℃ (calcd. 13.1%, absd. 12.7%). Compound 2 is thermally stable up to 350 ℃. The decomposition occurs in the temperature range 350-430 ℃. pseudorotaxanes 1 is less stable than pseudorotaxanes 2 due to the thermal decomposition of oxalate, while water clusters exhibit the same stability in both compounds 1 and 2.
The removal and re-adsorption of water in pseudorotaxanes 1 and 2 were investigated by X-ray powder diffraction (XRD) (Figs. S4 and S5 in Supporting information). A low-angle peak disappeared after compound 1 was heated at 110 ℃ for 3 h in N2 atmosphere, which indicated that the major contribution to this peak comes from the structured water. Subsequently, the dehydrated sample was placed in a humid container at room temperature for 3 days, the XRD spectrum showed that some new peaks were observed instead of those disappeared peaks, indicating an irreversible change of the structure after rehydration. This might be attributed to the rehydration of water clusters, which leads to the generation of different structures. The XRD spectrum of dehydrated pseudorotaxane 2 was similar to that of compound 1. But the peak in the XRD pattern of the rehydrated sample restored after exposed to moisture, indicating that the architecture of the sample was regenerated after rehydration. Therefore, the loss of water molecules from the lattice led to the breakdown of the 3D structures. The result was significantly different from that of compound 1.
4. ConclusionTwo types of anion-water clusters, 2D-layered L18(8)14(8)8(4) type [(HC2O4)4(H2O)22]4- and 1D "W"-like T5(0)A2 type anion- water clusters [(C6H5SO3)(H2O)6]- were self-assembled with the induction of anion templates in the CB[6] supramolecular systems. Anion water clusters are affected by the factors of spatial structure: symmetry, locations of binding sites and structural rigidity. The present route demonstrated that the structure of anion with high symmetry, more dispersive stretching directions and more rigidity may induce tighter and more highly symmetrical water clusters. The majority of interactions were O...H and H...H interactions on the Hirshfeld surface (around 80%) in both compounds 1 and 2, which indicated that hydrogen bonding and hydrophobic interactions were the dominate drive forces in forming these supramolecular systems.
AcknowledgmentsWe gratefully acknowledge the financial support by the National Natural Science Foundation of China (Nos. 21202037 and 21401044) and Doctor Fund of Henan University of Technology (No. 2013BS066).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2015.12.003.
| [1] | D. Eisenberg, W. Kauzmannc, The Structure and Properties of Water, Oxford University Press, Oxford, UK, 1969. |
| [2] | P. Ball, H2O:a Biography of Water, Weidenfeld & Nicolson, London, 1999. |
| [3] | R. Ludwig, Water:from clusters to the bulk, Angew. Chem. (Ⅰ)nt. Ed. 40(2001) 1808-1827. |
| [4] | N. Pugliano, R.J. Saykally, Measurement of quantum tunneling between chiral isomers of the cyclic water trimer, Science 257(1992) 1937-1940. |
| [5] | U. Buck, F. Huisken, (Ⅰ)nfrared spectroscopy of size-selected water and methanol clusters, Chem. Rev. 100(2000) 3863-3890. |
| [6] | T. Head-Gordon, G. Hura, Water structure from scattering experiments and simulation, Chem. Rev. 102(2002) 2651-2670. |
| [7] | X.B. Wang, X. Yang, J.B. Nicholas, L.S. Wang, Bulk-like features in the photoemission spectra of hydrated doubly charged anion clusters, Science 294(2001) 1322-1325. |
| [8] | W.H. Robertson, E.G. Diken, E.A. Price, J.W. Shin, M.A. Johnson, Spectroscopic determination of the OH-solvation shell in the OH-·(H2O)n clusters, Science 299(2003) 1367-1372. |
| [9] | R. Custelcean, M.G. Gorbunova, A metal-organic framework functionalized with free carboxylic acid sites and its selective binding of a Cl(H2O)4- cluster, J. Am. Chem. Soc. 127(2005) 16362-16363. |
| [10] | C.K. Lam, F. Xue, J.P. Zhang, X.M. Chen, T.C.W. Mak, Hydrogen-Bonded anionic rosette networks assembled with guanidinium and C3-symmetric oxoanion building blocks, J. Am. Chem. Soc. 127(2005) 11536-11537. |
| [11] | A. Bakhoda, H.R. Khavasi, N. Safari, Discrete cubane-like bromide-water cluster, Cryst. Growth Des. 11(2011) 933-935. |
| [12] | M.A. Hossain, P. Morehouse, D. Powell, K. Bowman-James, Tritopic (Cascade) and ditopic complexes of halides with an azacryptand, (Ⅰ)norg. Chem. 44(2005) 2143-2149. |
| [13] | Q.H. Pan, R.J. Tian, S.J. Liu, et al.,[Co(NH3)6]2[Cd8(C2O4)11(H2O)4]·8H2O:a 5-connected sqp topological metal-organic framework co-templated by Co(NH3)63+ cation and (H2O)4 cluster, Chin. Chem. Lett. 24(2013) 861-865. |
| [14] | G.G. Luo, S.H. Wu, Z.H. Pan, Z.J. Xiao, J.C. Dai, Formation of different polymeric water clusters via organic anionic templates:more carboxylate groups used, more water molecules gathered, (Ⅰ)norg. Chem. Comm. 39(2014) 34-38. |
| [15] | G.G. Luo, H.B. Xiong, J.C. Dai, Syntheses, structural characterization, and properties of {[Cu(bpp)2(H2O)2] (tp)·7H2O} and {[Cu(bpp)2(H2O)](ip)·7H2O} complexes, New examples of the organic anionic template effect on induced assembly of water clusters (bpp=1, 3-Bis(4-pyridyl)propane, tp=Terephthalate, ip=(Ⅰ)sophthalate), Cryst. Growth Des. 11(2011) 507-515. |
| [16] | S. Ganguly, R. Mondal, Coordination driven self-assembly in Co(Ⅱ) coordination polymers displaying unprecedented topology, water cluster, chirality, and spincanted magnetic behavior, Cryst. Growth Des. 15(2015) 2211-2222. |
| [17] | (a) N. Nijem, P. Canepa, U. Kaipa, et al., Water cluster confinement and methane adsorption in the hydrophobic cavities of a fluorinated metal-organic framework, J. Am. Chem. Soc. 135(2013) 12615-12626;(b) H. (Ⅰ)sobe, S. Sato, E. Nakamura, Synthesis of disubstituted cucurbit[6] uril and its rotaxane derivative, Org. Lett. 4(2002) 1287-1289. |
| [18] | (a) H. Yin, G. Hummer, J.C. Rasaiah, Metastable water clusters in the nonpolar cavities of the thermostable protein tetrabrachion, J. Am. Chem. Soc. 129(2007) 7369-7377;(b) W.A. Freeman, W.L. Mock, N. Shih, Cucurbituril, J. Am. Chem. Soc. 103(1981) 7367-7368. |
| [19] | (a) S. Parthasarathy, A. Altuve, S. Terzyan, et al., Accommodating a nonconservative internal mutation by water-mediated hydrogen bonding between β-sheet strands:a comparison of human and rat type B (Mitochondrial) cytochrome b5, Biochemistry 50(2011) 5544-5554;(b) W.L. Mock, N. Shih, Organic ligand-receptor interactions between cucurbituril and alkylammonium ions, J. Am. Chem. Soc. 110(1988) 4706-4710. |
| [20] | J. Lagona, P. Mukhopadhyay, S. Chakrabarti, L. (Ⅰ)saacs, The Cucurbit[n]uril family, Angew. Chem. (Ⅰ)nt. Ed. 44(2005) 4844-4870. |
| [21] | E. Lee, J. Heo, K. Kim, A three-dimensional polyrotaxane network, Angew. Chem. (Ⅰ)nt. Ed. 39(2000) 2699-2701. |
| [22] | E. Lee, J. Kim, J. Heo, D. Whang, K. Kim, A two-dimensional polyrotaxane with large cavities and channels:a novel approach to metal-organic open-frameworks by using supramolecular building blocks, Angew. Chem. (Ⅰ)nt. Ed. 40(2001) 399-402. |
| [23] | S.G. Roh, K.M. Park, S. Sakamoto, K. Tamaguchi, K. Kim, Synthesis of a fivemembered molecular necklace:a 2+2 approach, Angew. Chem. (Ⅰ)nt. Ed. 38(1999) 637-641. |
| [24] | K.M. Park, S.Y. Kim, J. Heo, et al., Designed self-assembly of molecular necklaces, J. Am. Chem. Soc. 124(2002) 2140-2147. |
| [25] | M.V. Rekharsky, H. Yamamura, M. Kawai, et al., Sequential formation of a ternary complex among dihexylammonium, cucurbit[6] uril, and cyclodextrin with positive cooperativity, Org. Lett. 8(2006) 815-818. |
| [26] | Y. Liu, X.Y. Li, H.Y. Zhang, C.J. Li, F. Ding, Cyclodextrin-driven movement of cucurbit[7] uril, J. Org. Chem. 72(2007) 3640-3645. |
| [27] | X.Z. Sun, B. Li, J. Cao, et al., Pseudopolyrotaxanes of cucurbit[6] uril:a threedimensional network self-assembled by ClO4-(H2O)2- water clusters, Chin. J. Chem. 30(2012) 941-946. |
| [28] | S. Angelos, Y.W. Yang, K. Patel, J.F. Stoddart, J.(Ⅰ). Zink, pH-Responsive supramolecular nanovalves based on cucurbit[6] uril, Angew. Chem. (Ⅰ)nt. Ed. 47(2008) 2222-2226. |
| [29] | Y.W. Yang, Y.L. Sun, N. Song, Switchable host-guest systems on surfaces, Acc. Chem. Res. 47(2014) 1950-1960. |
| [30] | X.Z. Sun, B. Li, Q.B. Zhou, et al., Pseudopolyrotaxanes of cucurbit[6] uril:a novel three-dimensional network self-assembled by (H2O)3 clusters and Br-(H2O)3 anion clusters, Cryst. Growth Des. 8(2008) 2970-2974. |
| [31] | X.Z. Sun, B. Li, C.L. Xia, X.H. Zhou, H.B. Zhang, "Liquid-like" type (COO-)2(H2O)10 anion water clusters in three dimensional supramolecular structure of cucurbit[6] uril, CrystEngComm 14(2012) 8525-8529. |
| [32] | L.J. Barbour, G.W. Orr, J.L. Atwood, An intermolecular (H2O)10 cluster in a solidstate supramolecular complex, Nature 393(1998) 671-673. |
| [33] | R. Custalcean, C. Afloroaiei, M. Vlassa, M. Polverejan, Formation of extended tapes of cyclic water hexamers in an organic molecular crystal host, Angew. Chem. (Ⅰ)nt. Ed. 39(2000) 3094-3096. |
| [34] | S.K. Seth, (Ⅰ). Saha, C. Estarellas, et al., Supramolecular self-assembly of M-(Ⅰ)DA complexes involving lone-pair·π interactions:crystal structures, hirshfeld surface analysis, and DFT calculations[H2(Ⅰ)DA=iminodiacetic acid.M=Cu(Ⅱ), Ni(Ⅱ)], Cryst. Growth Des. 11(2011) 3250-3265. |
| [35] | M.A. Spackman, P.G. Byrom, A novel definition of a molecule in a crystal, Chem. Phys. Lett. 267(1997) 215-220. |
| [36] | S.K. Wolff, D.J. Grimwood, J.J. McKinnon, D. Jayatilaka, M.A. Spackman, Crystal-Explorer 3.1, University of Western Australia, Perth, Australia, 2007. |
 2016, Vol.27
2016, Vol.27 



