b. State Key Laboratory of Coordination Chemistry, Nanjing University, Nanjing 210093, China
Single-strained DNA (ssDNA)-templated silver nanoclusters (DNA-AgNCs) have received increasing attention due to their good biocompatibility and low toxicity [1, 2, 3]. Especially, C-rich oligonucleotides have been found to be capable of serving as ideal templates to form highly fluorescent DNA-AgNCs due to the specific nature of the C-Ag+ interaction [4, 5, 6]. Nucleic acids are also reported to interact with metal ions through nucleobases and the phosphodiester backbone [7]. The fluorescent properties of DNA-AgNCs can be modulated by the change of the conformation and the size. For example, silver nanoclusters constructed by the bifunctional oligonucleotide were used as fluorescence sensors to sense HepG-2 cells because the complementary sequence can assemble with DNA-AgNCs with the change of conformation and the size [8]. Recently, we found that the near-infrared fluorescent DNA-AgNCs constructed by the bifunctional oligonucleotide with parallel homoduplex conformation could recognize one isomer of boradiazaindacenes (BODIPY) based on the different energy transfer in the DNA-AgNCs-compounds conjugated system [9]. Cytosine bases can bind silver ions, which makes C-rich oligonucleotides a good template to synthesize fluorescent silver nanoclusters [10]. The sequence of CGGGCCAAGAGTGTGCTAAA (DNAT) has been used as forward nucleotides in the measurement of Glucose transporter 1 (GLUT1) by the Polymerase Chain Reaction (PCR) method [11]. The combination of C3T-rich aptamer and CGGGCCAAGAGTGTGCTAAA can form a new bifunctional oligonucleotide, which can be used as a template in the synthesis of high luminescence DNA-AgNCs. Herein, we report new types of assembled DNA-AgNCs and their responses to Fe(Ⅲ/Ⅱ) ions.
2. ExperimentalAll chemicals used were obtained from commercial sources and directly used without additional purification. Cytosine-(C)-rich DNA scaffolds were purchased from Sangon Inc. (Shanghai, China), the sequences of different DNA are shown in Table 1. Silver nitrates (AgNO3), citric acid, NaBH4 and Fe(NO3)3 were all obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Transmission electron microscopy (TEM) was performed at room temperature on a JEOL JEM-200CX transmission electron microscope using an accelerating voltage of 200 kV. The electronic absorption spectrum was recorded using a UV-2450 UV-vis spectrophotometer at room temperature. Fluorescence measurements were performed on a fluorescence spectrofluorometer Model CARY Eclipse (VARIAN, USA), a 1.0 cm quartz cell, slit width = 5 nm. The mixtures were subjected to fluorescence measurement. The fluorescence quenching constants were calculated according to the formula: Q = (F0 × F)/F0 (F0 is the original fluorescence intensity; F is the fluorescence intensity of the mixture).
|
|
Table 1 Oligonucleotides designed for use in this study. |
DNA-silver nanoclusters (DNA-AgNCs) were synthesized by combining DNA template and Ag+ solutions in a 10 mmol/L citrate buffer at pH = 5. The final concentration of AgNO3 and DNA is 6.4 μmol/L and 200 μmol/L, respectively. Then the mixture was heated to 72 ℃ and maintained for 3 min, followed by slowly cooling to room temperature. The whole cooling process is about 3 h. An aqueous solution of NaBH4 was added to give a final concentration of 2 BH4 -/Ag+ (molar ratio) at room temperature, and the resulting solution was vigorously shaken for 1 min and left staying for 3 days in the dark at 4 ℃ [8].
3. Results and discussion 3.1. Design and characterization of DNA-AgNCs and enhancer sequencesDNA-based silver nanoclusters (DNA1-AgNCs) were synthesized by using a template containing a C3T-rich scaffold (DNAC) and an enhance sequence (DNAT) (Table 1). It can be seen that the DNA1-AgNCs have the sizes of ca. 2-4 nm with an emission maximum at 663 nm (Fig. 1A and B). The UV-vis absorption peaks of the DNA1-AgNCs are at 410 nm and 600 nm (Fig. 1B). In order to investigate the key section of the DNAT on the emission of synthesized silver nanoclusters, DNA1 was divided into DNA2 (DNA2 = DNAC + CGGGTGT (GT are linking sites because GT can recognize CA in DNA3)) and DNA3 (CACTGT to replace CGGG in DNAT) (Table 1), and another kind of DNA-based silver nanoclusters (DNA2-AgNCs) was obtained. The sizes of DNA2- AgNCs are in the range of 8-10 nm (Fig. S1A in Supporting information). The DNA1-AgNCs and DNA2-AgNCs have the same emission maximum at 663 nm due to the same DNAC used as the template to stabilize the Ag clusters. The quantum yield (Φ) of DNA1-AgNCs and DNA2-AgNCs for the emission at 660 nm is 0.33 and 0.25, respectively, using Ru(bpy)3Cl2 as reference (Φ = 0.062), which indicates that DNA3 may be an enhancer nucleotide. This will provide an opportunity to synthesize functional DNA-based sliver nanoclusters with red emission by the assembly of DNA2-AgNCs with a multifunctional enhancer.

|
Download:
|
| Fig. 1.(A) A TEM image of DNA1-AgNCs. (B) UV-vis absorption spectrum and fluorescence spectra of DNA1-AgNCs: (a) the UV-vis absorption spectrum; (b) and (c) the excitation and emission fluorescent spectra, respectively (λex = 600 nm, λem = 663 nm). | |
It is interesting to find that once adding DNA3 (a modified DNAT) to the solution of DNA2-AgNCs, an enhanced emission at 663 nm was observed, and when the ratio of DNA3 and DNA2- AgNCs came to 1:1, the fluorescence was nearly stable (Fig. 2A), indicating that formation of luminescence DNA3-DNA2-AgNCs. To explore the key bases in DNA3, DNA4(no GAG containing sequence) and DNA5 (double GAG containing sequence) were designed (Table 1) and used as replacer of DNA3. Fluorescence enhancement was observed after the addition of DNA5 while DNA4 had negligible impact on the fluorescence of DNA2-AgNCs. Hence, this further confirms GAG is an emission enhancer of DNA2-AgNCs (Fig. S2 in Supporting information). Moreover, the fluorescent DNA3C-AgNCs, DNA4C-AgNCs and DNA5C-AgNCs were synthesized by using assemblies of DNAC and DNA3 (1:1) or DNAC and DNA4 (1:1) or DNAC and DNA5 (1:1), respectively, as templates. The emission wavelength of DNA3C-AgNCs, DNA4CAgNCs and DNA5C-AgNCs are 686 nm, 633 nm and 697 nm, respectively (Fig. S3). This indicates GAGGAG can also assemble with C3T-rich oligonucleotides for the construction of red emission DNAn-AgNCs.

|
Download:
|
| Fig. 2. (A) Emission spectra of the fluorescent DNA2-AgNCs (λex = 600 nm, λem = 663 nm, 2 μmol/L) in the presence of DNA3 (the concentration of DNA3 from the bottom: 0, 0.1, 0. 5, 1.0, 1.5, 2.0, 2.5 μmol/L); (B) the fluorescence quenching constants of the DNA-AgNCs (2 μmol/L) incubated with a concentration of 0.2 μmol/L Fe3+ and Fe2+ (I: DNA1-AgNCs, Ⅱ: DNA2-AgNCs, Ⅲ: DNA4C-AgNCs, IV: DNA5C-AgNCs). | |
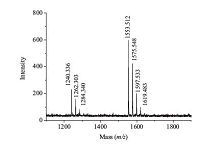
Results demonstrated DNA1-AgNCs are more sensitive to Fe3+ and Fe2+ ions than DNA2-AgNCs (Fig. 2B). This implies DNA3 may have some specific relationship with Fe(Ⅲ/Ⅱ) ions. Compared with DNA4C-AgNCs, DNA5C-AgNCs are more sensitive to Fe3+ or Fe2+ (Fig. 2B), which suggests that the GAG containing sequences may mediate the DNA-iron interplay. Because the DNA3 is an emission enhancer of DNA2-AgNCs, the effect of Fe3+ on the emission of DNA3-DNA2-AgNCs assemblies was studied. The fluorescence emission decreased when Fe3+ was added into the mixture of DNA3 and DNA2-AgNCs (Fig. S4 in Supporting information). In contrast, the 1:1 (molar ratio) mixture of DNA3 and Fe3+ showed no obvious influence on the fluorescence of DNA2-AgNCs (Fig. S4 in Supporting information). We deduce that the free Fe3+ ions can bind to the DNA3 section in the DNA3-DNA2-AgNCs system, leading to the deceased emission because guanine contains an imidazole ring which allows metal ions to coordinate [12]. This further confirms that GAG is the key factor to the interaction of free Fe3+ with DNA3-DNA2-AgNCs or DNA1-AgNCs. To confirm the combination of GAG and Fe3+, DNA6 was designed (Table 1). The main mass spectral peaks at m/z = 1=3.5 [(DNA6)5Fe2]6+ and 1240.3 [(DNA6)2Fe]3+ indicate that the main ratios of DNA6 and Fe3+ are about 5:2 and 2:1 (Fig. 3). The ESI-MS data demonstrate that the Fe3+ ions can bind with DNA6 forming a DNA6-Fe3+ complex. The nanoclusters (DNA1-AgNC) have a broad absorption band in 400-600 nm with maxima at 424 and 520 nm because of electronic transitions for small silver clusters, especially Ag2 and Ag3, are expected in this spectral region [13]. However, once adding Fe3+ ions into the solution of DNA1-AgNCs, λmax shifted from 424 to 395 nm (Fig. 4), and then the emission of DNA1- AgNCs following direct excitation of electronic bands of silver clusters decreased. These suggest that the Fe3+ can bind to sequence (DNAT) resulting in the effective aggregation of DNAn- Ag clusters (n = 1, 5c) and the formation of non-emission nanoclusters (DNAn-AgNC-Fe).
|
Download:
|
| Fig. 3.ESI-MS spectrum of DNA6-Fe3+ system. | |

|
Download:
|
| Fig. 4.UV-vis absorption spectra of: DNA1 (black line); DNA1-AgNCs (blue line); DNA1-AgNCs in the presence of Fe3+ (green line), Inset: DNA1 (black line); DNA1- AgNCs (blue line); DNA1-AgNCs in the presence of Fe3+ (green line). | |
New types of fluorescent silver nanoclusters were synthesized utilizing C-rich bifunctional nucleotides as templates. It is found that the GAG containing sequences assemble with DNAn-AgNCs, synthesized by C3T-rich nucleotides, producing new silver clusters with an enhanced red emission at about 660 nm. Besides, the synthesized silver nanoclusters with GAG containing sequences are sensitive to Fe3+ and Fe2+ ions due to the formation of nonemission DNA1-AgNCs-Fe nanoclusters. This will give a new path for the development of metal ion sensors based on the recognition of metal ions with the special sequence in DNA-Ag nanoclusters. Thus, GAG-containing nucleotides can be an enhancer for the emission of silver clusters with C3T-rich nucleotides and a mediator of the iron-clusters interplay.
AcknowledgmentsFinancial support of National Natural Science Foundation of China (No. 21271090) and Coordination Chemistry State key Laboratory Foundation of Nanjing University.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2015.12.013.
| [1] | T. Li, L.B. Zhang, J. Ai, S.J. Dong, E.K. Wang, (Ⅰ)on-tuned DNA/Ag fluorescent nanoclusters as versatile logic device, ACS Nano 5(2011) 6334-6338. |
| [2] | J.P. Yuan, W.W. Gou, E.K. Wang, Oligonucleotide stabilized silver nanoclusters as fluorescence probe for drug-DNA interaction investigation, Anal. Chim. Acta 706(2011) 338-342. |
| [3] | J. Sharma, H.C. Yeh, H. Yoo, J.H. Werner, J.S. Martinez, Silver nanocluster aptamers:in situ generation of intrinsically fluorescent recognition ligands for protein detection, Chem. Commun. 47(2011) 2294-2296. |
| [4] | Z.Z. Huang, F. Pu, Y.H. Lin, J.S. Ren, X.Q. Qu, Modulating DNA-templated silver nanoclusters for fluorescence turn-on detection of thiol compounds, Chem. Commun. 47(2011) 3487-3489. |
| [5] | J.J. Yin, X.X. He, X.K. Jia, K.M. Wang, F.Z. Xu, Highly sensitive label-free fluorescent detection of Hg2+ ions by DNA molecular machine-based Ag nanoclusters, Analyst 138(2013) 2350-2356. |
| [6] | P. Shah, A. Rørvig-Lund, S.B. Chaabane, et al., Design aspects of bright red emissive silver nanoclusters/DNA probes for microRNA detection, ACS Nano 6(2012) 8803-8814. |
| [7] | J.M. Obliosca, C. Liu, H.C. Yeh, Fluorescent silver nanoclusters as DNA probes, Nanoscale 5(2013) 8443-8461. |
| [8] | T.T. Zhao, Q.Y. Chen, C. Zeng, et al., Multi-DNA-Ag nanoclusters:reassembly mechanism and sensing the change of H(Ⅰ)F in cells, J. Mater. Chem., B 1(2013) 4678-4683. |
| [9] | T.T. Zhao, Q.Y. Chen, P.D. Wang, Z.P. Chen, A DNA-Ag cluster as a sensor for BOD(Ⅰ)PY isomers and HepG-2 cells, RSC Adv. 4(2014) 10390-10394. |
| [10] | H.C.Yeh, J.Sharma, J.J.Han, J.S.Martinez, J.H.Werner,ADNA-silvernanoclusterprobe that fluoresces upon hybridization, Nano Lett. 10(2010) 3106-3110. |
| [11] | K. Reinicke, P. Sotomayor, P. Cisterna, et al., Cellular distribution of Glut-1 and Glut-5 in benign and malignant human prostate tissue, J. Cell. Biochem. 113(2012) 553-562. |
| [12] | C.L. Guo, J. (Ⅰ)rudayaraj, Fluorescent Ag clusters via a protein-directed approach as a Hg(Ⅱ) ion sensor, Anal. Chem. 83(2011) 2883-2889. |
| [13] | J.T. Petty, J. Zheng, N.V. Hud, R.M. Dickson, DNA-templated Ag nanocluster formation, J. Am. Chem. Soc. 126(2004) 5207-5212. |
 2016, Vol.27
2016, Vol.27 



