b. Shanghai Engineering Research Center of Pharmaceutical Process, Shanghai 201203, China;
c. Shandong Provincial Hospital Affiliated to Shandong University, Jinan 250021, China
Stroke has been the second-leading cause of death and a main cause of neurological disability in the world over the past decade [1]. In particular,ischemic stroke,which accounts for more than 80% of all strokes,carries a high morbidity and mortality. Furthermore,strokes are likely to significantly increase in prevalence given its associated risk factors,such as obesity,diabetes,and hypertension [2, 3]. However,available drugs are very limited,and some of them suffer from poor efficacy and high toxicity. Clinically,intravenous thrombolysis and mechanical thrombectomy are current strategies for treating acute ischemic stroke [4],and tissue plasminogen activator (rt-PA) is the only FDA-approved drug for thrombolysis treatment. Unfortunately,it is used in less than 5% of patients due to its narrow therapeutic window and complexity of administration [5]. Edaravone [6, 7, 8, 9] and Butylphthalide [10, 11, 12, 13, 14] (Fig. 1) were approved by CFDA for acute treatment as neurointervention therapy and cerebral microcirculation improvement drug in ischemic stroke patients in 2005 and 2002,respectively. They exhibited neuroprotective effects and antithrombotic activity in cerebral infarction and acute stroke,but the potency is limited unless administered with other antistroke drugs. Most critically,the penumbra (a peripheral zone of focal cerebral ischemia that is damaged but potentially reversible) transfers to irreversible damage within a few hours [15, 16]. Hence,there is an emergent need for development of novel effective neuroprotective agents.
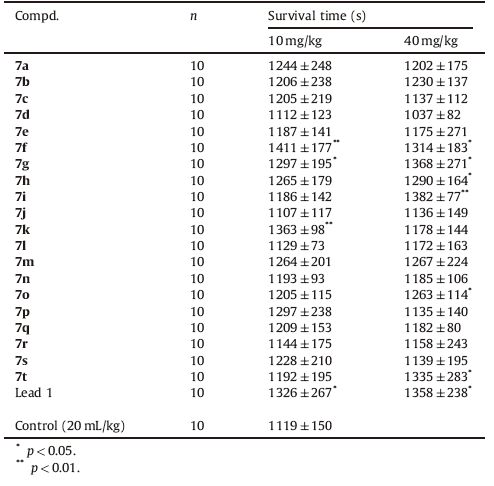
Previous studies have showed that aralkyl dicarbonyl piperazine compound 1 (Fenazinel,Fig. 1) exhibited potent anti-ischemic stroke activity as neuroprotective therapy,and was developed into clinical trials [17, 18, 19, 20, 21]. In order to investigate the structure-activity relationships (SARs),a series of novel dicarbonyl piperazine derivatives (Fig. 2) were synthesized and evaluated on their neuroprotective activity via oxygen-glucose deprivation test in neuron-like PC12 cells,a hypoxia tolerance model in mice,and a focal cerebral ischemia model in rats.
|
Download:
|
| Fig. 1. Chemical structures of representative anti-ischemic stroke agents and the lead compound 1. | |

|
Download:
|
| Fig. 2.Design rational of the targeted compounds 7a-t. | |
All the starting materials were obtained from commercial sources and used directly without further purification. All the reactions were monitored by TLC analysis,carried out on silica gel plates HSGF254 (Yantai Jiangyou Chemical,China). Melting points (uncorrected) were determined on a Shanghai Jingmi WRR apparatus. 1H NMR spectra were recorded on a Varian INOVA- 400 NMR spectrometer,using TMS as an internal standard and DMSO as solvents. Chemical shifts (δ values) and coupling constants (J values) are given in ppm and Hz,respectively. ESI mass spectra were performed on a Finning-MAT 212 spectrometer. Silica gel column chromatography was performed with Silica gel 60G (Qingdao Haiyang Chemical,China).
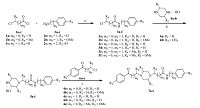
The general synthetic routes of compounds 7a-t are outlined in Schemes 1 and 2. Compounds 2a-c were acylated by chlorosubstituted acyl chloride in CHCl3 to give compounds 3a- f. Intermediates 5a-i were obtained by alkylation of 4a or b with 3a-f in EtOH via refluxing,which was subsequently alkylated with 6a-e in acetone at presence of K2CO3 to afford the targeted compounds 7a-r in 79%-87% yield. Compounds 7s and t were synthesized via two alkylation steps. 2,5-Dimethylpiperazine (8) was alkylated with 6d-e in presence of KOH and CTBA with molar ratio of 3.5:1:1:0.01,and then alkylated with 3a and b in MeOH at presence of K2CO3 to afford 7s and t in 80%-85% yield.
|
Download:
|
| Scheme 1.Synthetic route of compounds 7a-r. Reagents and conditions: (a)CHCl3,0 ℃,2 h,60%-75%; (b) EtOH,reflux,1.5 h,75%-85%; (c)K2CO3,acetone,reflux,12 h,79%-87%. | |
Structures of targeted compounds 7a-t are illustrated in Table 1. All the compounds 7a-t were evaluated on their neuroprotective activity via in vitro (oxygen-glucose deprivation test in PC12 cells) and in vivo assays (hypoxia tolerance model in mice and focal cerebral ischemia model in rats). The detailed synthetic procedure,in vitro and in vivo biological evaluations,and identification data of target compounds 7a-t,characterized by 1H NMR and mass spectrometry techniques are shown in the Supporting information.
|
|
Table 1 Structures of the targeted compounds of 7a-t. |
The design rationale for the targeted compounds is depicted in Fig. 2. Methyl,methylene or methoxy were introduced to methylene,piperazine,middle of actetyl and piperazine-N,as well as both terminal phenyl of the lead compound 1,respectively. Twenty new compounds 7a-t (Schemes 1 and 2) were synthesized and characterized.

|
Download:
|
| Scheme 2.Synthetic route of compounds 7s and t. Reagents and conditions: (i) KOH,CTBA,EtOH,reflux,1 h,80%; (ii) K2CO3,MeOH,reflux,12 h,80%-85%. | |
In vitro oxygen-glucose deprivation (OGD) test data was shown in Table 2. The MTT assays indicated that treatment with OGD for 2 h effectively inhibited the growth of PC12 cells,and the survival rate was 72.35%. Compared to the OGD for 2 h treated group,most of the targeted compounds plus OGD for 2 h treated group could significantly increase the survival rate in a dose-dependent manner and the rate was higher than that of OGD group. Methyl or methoxy introduction in methylene or 4-phenyl as compounds 7a,7c,7f and 7g exhibited higher survival rate than the lead compound 1,while methylene insertion led to a decrease or lose of survival rate as the data revealed for compounds 7b,7d and 7e. Compounds 7o containing 2,5-dimethylpiperazin moiety displayed higher survival rate than compound 1. The above compounds showed 85%-97% viable rate at three levels of concentrations,suggesting potential neuroprotective activity by means of relieving OGD damage.
|
|
Table 2 In vitro neuroprotective activity in oxygen-glucose deprivation test in PC12 cells. |
In vivo neuroprotective activity data in hypoxia tolerance model in mice and focal cerebral ischemia model in rats was shown in Tables 3 and 4. Hypoxia tolerance assay showed that compounds 7a,7f,7g,7k and 7o could significantly prolong the survival time of mice under hypoxic condition at dose of 10 and 40 mg/kg than the control group,and were comparable with lead compound
|
|
Table 3 In vivo neuroprotective activity in hypoxia tolerance model in mice. |
|
|
Table 4 In vivo neuroprotective activity in focal cerebral ischemia model in rats (n = 3). |
infarction in brain after ischemia for 24 h was investigated. Compounds 7f,7k,and 7o could significantly reduce the ratio of cerebral infarction (5.43%-5.99%) compared with the control group (10.28%) and then improve the neurological function. In particular,compound 7o showed excellent anti-stroke activity,which is a potential candidate for the development of neuroprotective agents.
4. ConclusionIn summary,a series of novel dicarbonyl piperazine derivatives were designed,synthesized,and evaluated on their neuroprotective activity. The SARs revealed that methyl or methoxy monointroduction was favorable for neuroprotective activity,while methylene introduction was not. It is worth mentioning that compound 7o,containing a 2,5-dimethylpiperazin moiety,showed good anti-ischemic stroke activity in vitro and in vivo,which provided a good starting point for a potential candidate for the development of neuroprotective agents.
AcknowledgmentThis work was supported by Shanghai Natural Science Foundation (No. 12ZR1450100).
Appendix A. Supplementary dataSupplementary material related to this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.11.002.
| [1] | World Health Organization (WHO). The Top 10 Leading Causes of Death in the World, 2000 and 2012. Global Health Observatory Data, . |
| [2] | D.A. Bennett, R.V. Krishnamurthi, S. Barker-Collo, et al., The global burden of ischemic stroke:findings of the GBD 2010 study, Global Heart 9(2014) 107-112. |
| [3] | A.S. Go, D. Mozaffarian, V.L. Roger, et al., Heart disease and stroke statistics-2013 update:a report from the American Heart Association, Circulation 127(2013) e6-e245. |
| [4] | R. Mikulik, N. Wahlgren, Treatment of acute stroke:an update, J. (Ⅰ)nt. Med. 278(2015) 145-165. |
| [5] | P. Lyden, Thrombolytic therapy for acute stroke-not a moment to lose, N. Engl. J. Med. 359(2008) 1393-1395. |
| [6] | H. Yoshida, H. Yanai, Y. Namiki, et al., Neuroprotective effects of edaravone:a novel free radical scavenger in cerebrovascular injury, CNS Drug Rev. 12(2006) 9-20. |
| [7] | S. Okuyama, M. Morita, A. Sawamoto, et al., Edaravone enhances brain-derived neurotrophic factor production in the ischemic mouse brain, Pharmaceuticals 8(2015) 176-185. |
| [8] | T. Wada, H. Yasunaga, R. (Ⅰ)nokuchi, et al., Effects of edaravone on early outcomes in acute ischemic stroke patients treated with recombinant tissue plasminogen activator, J. Neurol. Sci. 345(2014) 106-111. |
| [9] | K. Kikuchi, E. Tanaka, Y. Murai, S. Tancharoen, Clinical trials in acute ischemic stroke, CNS Drugs 28(2014) 929-938. |
| [10] | C.L. Liu, S.J. Liao, J.S. Zeng, et al., dl-3-n-Butylphthalide prevents stroke via improvement of cerebral microvessels in RHRSP, J. Neurol. Sci. 260(2007) 106-113. |
| [11] | L.H. Zhang, W.H.A. Yu, Y.X.J. Wang, et al., DL-3-n-Butylphthalide, an anti-oxidant agent, prevents neurological deficits and cerebral injury following stroke per functional analysis, magnetic resonance imaging and histological assessment, Curr. Neurovasc. Res. 9(2012) 167-175. |
| [12] | J.M. Li, Y. Li, M. Ogle, et al., DL-3-n-Butylphthalide prevents neuronal cell death after focal cerebral ischemia in mice via the JNK pathway, Brain Res. 1359(2010) 216-226. |
| [13] | L.Y. Cui, X.Q. Liu, Y.C. Zhu, et al., Effects of dl-3-butylphthalide on treatment of acute ischemic stroke with moderate symptoms:a multi-center, randomized, double-blind, placebo-control trial, Chin. J. Neurol. 38(2005) 251-254. |
| [14] | L.Y. Cui, Y.C. Zhu, S. Gao, et al., Ninety-day administration of dl-3-n-butylphthalide for acute ischemic stroke:a randomized, double-blind trial, Chin. Med. J. 126(2013) 3405-3410. |
| [15] | E.H. Lo, A new penumbra:transitioning from injury into repair after stroke, Nat. Med. 14(2008) 497-500. |
| [16] | W.D. Heiss, (Ⅰ)schemic penumbra:evidence from functional imaging in man, J. Cereb. Blood Flow Metab. 20(2000) 1276-1293. |
| [17] | J.Q. Li, L.Y. Huang, Y.Y. Xia, et al., Synthesis of aroylpiperazine derivatives and their anti-cerebral anoxia, anti-cerebral ischemia biological activities, Chin. J. Med. Chem. 16(2006) 6-14. |
| [18] | L.L. Jin, Y.C. Sheng, Y. Zhong, P. Zhu, Y.Y. Xia, Relation between therapeutic effects and administration time of fenazinel dihydrochloride on focal cerebral ischemia injury in rats, Chin. J. Pharm. 5(2008) 356-358. |
| [19] | H. Jiang, Z.W. Yang, Y.Y. Chen, Z.A. Lu, F.M. Shen, Effective dose of fenazinel dihvdrochloride in the treatment of cerebral infarction in rats, Guide China Med. 7(2009) 77-78. |
| [20] | D.J. Li, J.Q. Li, L.Y. Huang, et al., Protective effects of fenazinel dihydrochloride on focal cerebral ischemic injury in rats, Chin. Pharmacol. Bull. 25(2009) 716-720. |
| [21] | T. Zhao, Z. Wei, F.M. Shen, Protective effects of fenazinel dihydrochloride against stroke in stroke-prone spontaneously hypertensive rats, Acad. J. Second Mil. Med. Univ. 12(2011) 1282-1285. |
 2016, Vol.27
2016, Vol.27