b. University of Chinese Academy of Sciences, Beijing 100049, China;
c. Institute of Medical Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100193, China
Plant-parasitic nematodes have been one of the most notorious plant pathogens worldwide [1, 2]. Thousands of crops and trees are susceptible and the disease caused by the phytonematodes results in billions of agricultural losses annually [3]. Chemical methods,combined with agriculture practice,have been the primary way for the nematode control [4, 5, 6, 7]. To lessen environmental toxicity,pesticide residues and nematode resistance,the development of new control substitutes has become an urgent and challenging task [8]. Natural products and their derivatives provide a promising treasury for the identification of modern pesticides [9]. As an alternative to a large screening program for the identification of new active materials,a rational program of structural modification of known active compounds can be more efficient and equally useful.
Coumarins are widely available promising natural compounds for modification due to their broad bioactivities [10, 11, 12]. Some simple coumarins,furocoumarines and dicoumarolums,display excellent nematicidal activity and their skeletons have drawn interest for the development of efficient nematicides [13, 14]. In our previous study,7-hydroxycoumarin was isolated from Stellera chamaejasme and had been discovered to show nematicidal activity against Bursaphelenchus xylophilus and Bursaphelenchus mucronatus. In recent years,the structure-activity relationships (SAR) of hydroxycoumarin derivatives have been intensively conducted and indicated that C4 and C7 position modifications in the backbone could enhance their tumor cytotoxicity,termiticidal and bactericidal activities [15, 16, 17, 18].
Therefore,we were prompted to design and develop coumarinbased nematicides by modification at these positions (Fig. 1). In a pilot study,we coupled 4-hydroxycoumarin (1) and 7-hydroxy- 4-methylcoumarin (2) with alkyl bromides,and a significant difference in activity was observed. Based on this,we proposed to further develop modifications of 4-hydroxycoumarin (1) and 7-hydroxy-4-methylcoumarin (2) by coupling coumarin with functional moieties at the C4 and C7 hydroxyls,and five phytonematodes were applied to evaluate their nematicidal activities.
|
Download:
|
| Fig. 1.Structures of coumarins and their modification sites. | |
All starting chemicals were of analytical reagent and used without purification. Reactions were monitored by precoated TLC plates (Silica Gel 60 F254. Qingdao Haiyang Chemical Co.,Ltd.,Qingdao,China),and the spots were visualized by ultraviolet (UV) illumination. Silica gel (200-300 mesh) (Qingdao Haiyang Chemical Co.,Ltd.) was used for column chromatography. Melting points were tested with a X-4 melting point apparatus (Beijing Tech Instrument Co.,Ltd.,China). The structural 1H NMR and 13C NMR spectra were performed on a Bruker AM-400BB instrument (Bruker,Karlsruhe,Germany) with TMS as internal standard,operating at 400 MHz. The chemical shift values are on a δ scale and the coupling constant values (J) are in Hertz. ESI-HRMS was recorded using a Bruker micrOTOF-Q II.
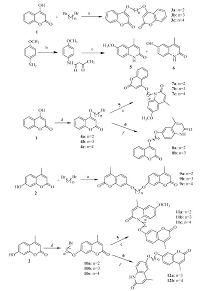
2.1. Synthesis of target compounds (3a-12b)The general synthesis is illustrated in Scheme 1. Compounds 3a-c were prepared by the reaction of 4-hydroxycoumarin (1) with 1,2-dibromoethane,1,3-dibromopropane and 1,4-dibromobutane,respectively,in the presence of an alkaline catalyst [19]. Compounds 9a-c were similarly prepared with the dibromides. 6-Methoxy-4-methylquinolone (5) and 6-hydroxy-4-methylquinolone (6) were synthesized via a Knorr reaction of ethyl acetoacetate with anisidine in the presence of H2SO4 [20]. The bromoalkoxy derivatives of 4-hydroxycoumarins (4a-c) and 7-hydroxy-4-methylcoumarins (10a-c) were prepared through the bromoalkylation of 4-hydroxycoumarin (1) and 7-hydroxy-4- methylcoumarin (2) with 1,2-dibromoethane,1,3-dibromopropane and 1,4-dibromobutane,respectively. N-Alkylation is classically realized with halogen derivatives under alkaline condition,but with simply potassium hydroxide as a catalyst,the coupling reaction between alkyl bromides,coumarins (4a-c) and 6- methoxy-4-methylquinolone (5) was unsuccessful. Finally,a complex catalyst system of KOH,KI and tetrabutyl ammonium bromide (TBAB) was developed to prepare compounds 7a-c in high yield. Compounds 11a-c were then prepared by the reaction between 6-methoxy-4-methyl-quinolone (5) and 7-bromoalkoxy- 4-methylcoumarins (10a-c). 6-Hydroxy-4-methylquinolone (6) coupled with appropriate 4-bromoalkoxycoumarins (4a and b) in the presence of K2CO3,KI and TBAB to yield compounds 8a and 8b,and the reaction with 7-bromoalkoxy-4-methylcoumarins (10b and c) gave 12a and b. The coumarin analogs were analyzed by 1H NMR,13C NMR and HR-ESI-MS. The purity of all test compounds was above 95% (determined by HPLC). All the compounds were characterized and the data was listed in Supporting information. Here,compound 11b was taken as an example to show the typical structure of coumarin analogs.
|
Download:
|
| Scheme 1.Synthesis of coumarin derivatives. Reagents and conditions: (a) K2CO3/KOH/acetone,reflux; (b) ethyl acetoacetate,reflux; (c) 80% H2SO4,95 ℃; (d) K2CO3/acetone,reflux; (e) KOH/KI/TBAB/methylbenzene,90 ℃; (f) K2CO3/KI/TBAB/methylbenzene,90 ℃. | |
4-Methyl-7-[3-(6-methoxy-4-methylquinolin-2-on-1-yl)propyloxy] benzopyran-2-one (11b): White solid,yield 63%. mp 184- 185 ℃. Its molecular formula was determined to be C24H23NO5 from the HRESIMS data at m/z 406.2 [M + H]+. 1H NMR (400 MHz,CDCl3): δ 2.32-2.35 (m,2H,H-12),2.38 (s,3H,CH3-4'),2.58 (s,3H,CH3-4),3.92 (s,3H,OCH3-6),4.23 (t,2H,J =6.0 Hz,H-13),4.63 (t,2H,J =6.0 Hz,H-11),6.12 (s,1H,H-3'),6.76 (s,1H,H-3),6.83 (d,1H,J =2.4 Hz,H-8'),6.87 (dd,1H,J =8.8 Hz,2.4 Hz,H-6),7.14 (d,1H,J =2.4 Hz,H-8),7.25-7.28 (m,2H,H-7',H-5),7.46 (d,1H,J = 8.8 Hz,H-5'). 13C NMR (100 MHz,CDCl3): δ 18.68,18.89,28.95,30.95,=.97,65.46,101.43,103.39,111.91,112.07,112.73,113.12,113.53,120.26,125.47,125.57,
125.93,128.95,152.54,1=.29,156.04,160.44,161.34,162.07. The 1H NMR spectrum showed the two methyl protons and one methoxyl protons. The protons at d 6.12-7.46 indicated the moieties of coumarin and quinolone. The presence of the protons at δ 2.32-2.35 (H-12),4.23 (H-13) and 4.63 (H-11) revealed the existence of linker between the two moieties. The results of 13C NMR were in accordance with that of 1H NMR spectrum. Therefore,the compound 11b was elucidated as 4-Methyl-7-[3-(6-methoxy-4-methylquinolin-2-on-1-yl)propyloxy]- benzopyran-2-one.
Five prevalent nematodes,Meloidogyne incognita,Ditylenchus destructor,Bursaphelenchus xylophilus,Bursaphelenchus mucronatus and Aphelenchoides besseyi,were used to evaluate the nematicidal spectrum and potential of the synthesized compounds. B. xylophilus and B. mucronatus,isolated from Pinus massoniana,were supplied by Dr. Han Zhengmin (College of Forest Resources and Environment,Nanjing Forestry University). M. incognita,D. destructor and A. besseyi were provided by Dr. Lin Maosong (College of Plant Protection,Nanjing Agricultural University). D. destructor was grown on potato dextrose Agar (PDA) media containing a strain of Fusarium solani in 9-cm Petri dishes.
M. incognita,B. xylophilus,B. mucronatus and A. besseyi were cultured on PDA media with Botrytis cinerea Pers. All nematodes were stored at 28 ℃ and subcultured before bioassay. With successive observing under a microscope,J2s were collected and suspended in distilled water for the experiments. The tested compounds were dissolved in dimethylsulfoxide (DMSO) to obtain stock solutions,which were diluted with distilled water containing Tween-20 to prepare working solutions. 300 mL of dilutions and J2s suspension (containing about 60-80 J2s) were added into 24-well plates within 24 h. The final concentrations of DMSO and Tween-20 were kept under 0.5% and 0.05% of volume in each well,at which concentration levels,the motility of nematodes exposed was similar to those of a blank control. Distilled water and a solution of DMSO and Tween-20,at concentrations equivalent to those in the treatment wells,was used as a control. Abamectin was used as a positive control [21]. The plates were covered and parafilmed to prevent evaporation,and then incubated in the dark at 28 ℃ for 72 h. Juveniles were delivered to clean water and observed with a microscope at 40-magnification (Shanghai Optical Instrument Factory,Shanghai,China). Those immotile in a straight or "L" shape and not recovering in clean water were defined as dead. (Fig. 2) The experiment was conducted twice with three replicates. The values were determined as percentage corrected mortality (-standard deviation) according to the Schneider- Orelli formula:
Corrected mortality% = [(mortality% in treatment -mortality% in control)/(100 -mortality% in control)] × 100.
The compounds with strong activity were tested further under a series of concentrations to calculate their LC50 values. Probit analysis was used to estimate LC50 [22, 23].

|
Download:
|
| Fig. 2.Representative pictures of five plant-parasitic nematodes immersed in the solution of compound 9a at the concentration of 100 mmol/L for 72 h (observed with microscope at 100-magnification). | |
The target coumarin analogs were efficiently prepared in the presence of the complex catalytic system. Their nematicidal activity was evaluated under a series of concentration and the LC50 values were calculated (Table 1). As predicted,the modification on the hydroxyl at C4 and C7 positions led to the identification of promising lead compounds. Among the derivatives,compounds 7b,9a,10c and 11c showed significant strong nematicidal activity,with a broad spectrum against all tested nematodes. Compound 10c was the most effective with lowest LC50 values against M. incognita (5.1 mmol/L),D. destructor (3.7 mmol/L) and B. mucronatus (6.4 mmol/L),B. xylophilus (2.5 mmol/L) and A. besseyi (3.1 mmol/L) respectively. The rest of the derivatives exhibited moderate or weak nematicidal activity. Clearly,the type and position of the substituents were intensively responsible for the activity expression. Different substituents resulted in varied activity. When the coumarin moiety was replaced with that of a quinolone,a change of activity occurred. For the C4 position,substitution of the coumarin moieties with quinolone groups significantly increased (7b) or decreased (7a and c) the nematicidal activity. For the C7 position,compounds 9a-c showed significantly different activity compared with their quinolone derivatives (11a- c). Beside the effect of a particular functional group,the position effect was also significant. Most 7-hydroxy-4-methylcoumarin derivatives showed improved nematicidal activity,whereas only one among all the 4-hydroxycoumarin derivatives (7b) exhibited better activity. Thus,the C7 hydroxyl is more likely to be a better modification site for the preparation of promising compounds.
|
|
Table 1 Nematicidal activity of coumarin analogs against five plant-parasitic nematodes (calculated at 72 h). |
It is notable that the length of linkers between two terminal moieties was intensively responsible for the activity. For the linkers between two 4-hydroxycoumarins (1),the change of chain length hadlittle impact onthe activity of compounds (3a-c),with 3aand 3b exhibiting none activity and 3c just showing weak nematicidal activity to specific species. Three-carbon chain lengths (7b) were most active among the linkers between 4-hydroxycoumarin (1) and quinolone,and four-carbon atom lengths (7c) induced the loss of activity. For the coupling of two 7-hydroxy-4-methylcoumarins (2),the chain length of two carbon atoms (9a) was optimum,and chain lengths of three carbon atoms (9b) or four (9c) resulted in the great loss of activity. However,concerning to compounds 10-12,it was found a positive correlation between linker lengths and activities among quinolone coupled 7-hydroxy-4-methylcoumarins (11a-c).
Thiswasnot observedamongcompounds10a-c,but they all showed strong activities with four carbon chains. The chain length had little influence on the activity expression of compounds 12a and b. Itwas worth noting that brombutyl substituted 7-hydroxy-4-methylcoumarin (10c) exerted outstanding nematicidal activity,whereas bromopropyl (10b) and bromoethyl (10a) derivatives showedweak activity,which suggested that the four-carbon chain lengthwas the most active.Aboutmono-coumarin analogs andbi-coumarinones,it was found that,as a mono-coumarin analog,brombutyl coumarin (10c) exhibited better activities thanits bi-coumarin analog (9c),but the different results were observed between bromoethyl analog (10a) and bi-coumarin one (9a). This indicated that the better activity of compound 10c was determined by the combination of coumarinmoiety,bromoatoms and butyl chain. Taken together,the length of the alkyl chain was a key factor for the activity,but the optimumlengthwas notfixedandwasdependeduponthemoiety to which it was linked.
4. ConclusionIn summary,a series of coumarin-based compounds were designed and synthesized with a targeted derivatization of the C4 and C7 hydroxyl groups. Their activity against five prevalent nematodes was systematically investigated to reveal structure- activity relationship (SAR). The results showed that the expression of activity was determined by a combination of carbon chain link lengths and functional moieties,and that an optimum link chain length has been established. Compounds 7b,9a,10c and 11c exhibited the most efficient broad spectrum activity and could be promising for development as novel nematicides.
AcknowledgmentsWe thank Dr Frank Stermitz for his valuable help in English editing. The authors are thankful to the National Natural Science Foundation of China (Nos. 31070386,21302195 and 31300290),135 Key Cultivation Program of the Chinese Academy of Sciences,and the Province-Academy Cooperation Program of Henan Province of China (No. 102106000021) for financial support. Appendix A. Supplementary data Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2016.01.029.
| [1] | N.G. Ntalli, P. Caboni, Botanical nematicides:a review, J. Agric. Food. Chem. 60(2012) 9929-9940. |
| [2] | J.T. Pechacek, T.M. Bargar, M.R. Sabol, (Ⅰ)nhibition of nematode induced root damage by derivatives of methylenecyclopropane acetic acid, Bioorg. Med. Chem. Lett. 7(1997) 2665-2668. |
| [3] | D.J. Chitwood, Research on plant-parasitic nematode biology conducted by the United States Department of Agriculture-Agricultural Research Service, Pest. Manag. Sci. 59(2003) 748-753. |
| [4] | T.C. Thoden, M. Boppré, J. Hallmann, Effects of pyrrolizidine alkaloids on the performance of plant-parasitic and free-living nematodes, Pest. Manag. Sci. 65(2009) 823-830. |
| [5] | (Ⅰ).O. Giannakou, (Ⅰ).A. Anastasiadis, S.R. Gowen, et al., Effects of a non-chemical nematicide combined with soil solarization for the control of root-knot nematodes, Crop. Prot. 26(2007) 1644-1654. |
| [6] | D.J. Chitwood, S.L.F. Meyer, Phytochemically based nematode control:opportunities and challenges, J. Nematol. 46(2014), 145-145. |
| [7] | J. Kearn, E. Ludlow, J. Dillon, et al., Fluensulfone is a nematicide with a mode of action distinct from anticholinesterases and macrocyclic lactones, Pestic. Biochem. Phys. 109(2014) 44-57. |
| [8] | S.M. Seo, J. Kim, S.H. Koh, et al., Nematicidal activity of natural ester compounds and their analogues against pine wood nematode, Bursaphelenchus xylophilus, J. Agr. Food. Chem. 62(2014) 9103-9108. |
| [9] | D.J. Chitwood, Phytochemical based strategies for nematode control, Annu. Rev. Phytopathol. 40(2002) 221. |
| [10] | M.E. Riveiro,N.De Kimpe, A. Moglioni, et al., Coumarins:old compounds with novel promising therapeutic perspectives, Curr. Med. Chem. 17(2010) 1325-1338. |
| [11] | B.R. Vijay Avin, P. Thirusangu, V. Lakshmi Ranganatha, et al., Synthesis and tumor inhibitory activity of novel coumarin analogs targeting angiogenesis and apoptosis, Eur. J. Med. Chem. 75(2014) 211-221. |
| [12] | M.E. Riveiro, D. Maes, R. Vázquez, et al., Coumarins:old compounds with novel promising therapeutic perspectives, Bioorg. Med. Chem. 17(2009) 6547-6559. |
| [13] | H. Cui, H. Jin, Q. Liu, et al., Nematicidal metabolites from roots of Stellera chamaejasme against Bursaphelenchus xylophilus and Bursaphelenchus mucronatus, Pest. Manag. Sci. 70(2014) 827-835. |
| [14] | Z. Yang, Z. Yu, L. Lei, et al., Nematicidal effect of volatiles produced by Trichoderma sp, J. Asia-Pac. Entomol. 15(2012) 647-650. |
| [15] | K. Takaishi, M. (Ⅰ)zumi, N. Baba, et al., Synthesis and biological evaluation of alkoxycoumarins as novel nematicidal constituents, Bioorg. Med. Chem. Lett. 18(2008) 5614-5617. |
| [16] | S. Lee, K. Sivakumar, W.S. Shin, et al., Synthesis and anti-angiogenesis activity of coumarin derivatives, Bioorg. Med. Chem. Lett. 16(2006) 4596-4599. |
| [17] | M. Kawase, H. Sakagami, K. Hashimoto, et al., Structure-cytotoxic activity relationships of simple hydroxylated coumarins, Anticancer. Res. 23(2003) 3243-3246. |
| [18] | M. Adfa, Y. Hattori, T. Yoshimura, et al., Antitermite activity of 7-alkoxycoumarins and related analogs against Coptotermes formosanus Shiraki, (Ⅰ)nt. Biodeterior. Biodegrad. 74(2012) 129-135. |
| [19] | Y. Chen, S.L. Wang, X.Q. Xu, et al., Synthesis and biological investigation of coumarin piperazine (piperidine) derivatives as potential multireceptor atypical antipsychotics, J. Med. Chem. 56(2013) 4671-4690. |
| [20] | N. Priya, A. Gupta, K. Chand, et al., Characterization of 4-methyl-2-oxo-1, 2-dihydroquinolin-6-yl acetate as an effective antiplatelet agent, Bioorg. Med. Chem. Lett. 18(2010) 4085-4094. |
| [21] | Q.L. Dang, W.K. Kim, C.M. Nguyen, et al., Nematicidal and antifungal activities of annonaceous acetogenins from Annona squamosa against various plant pathogens, J. Agr. Food. Chem. 59(2011) 11160-11167. |
| [22] | P. Caboni, L. Tronci, B. Liori, et al., Tulipaline A:structure-activity aspects as a nematicide and V-ATPase inhibitor, Pestic. Biochem. Phys. 112(2014) 33-39. |
| [23] | J.O. Kong, S.M. Lee, Y.S. Moon, et al., Nematicidal activity of plant essential oils against Bursaphelenchus xylophilus (Nematoda:Aphelenchoididae), J. Asia-Pac. Entomol. 9(2006) 173-178. |
 2016, Vol.27
2016, Vol.27