b. Medicinal Chemistry and Drug Discovery Research Laboratory, Pharmacy Group, Birla Institute of Technology and Science-Pilani, Hyderabad Campus, Jawahar Nagar 500078, Telangana, India
Despite the availability of strong and powerful antitubercular drugs,the oldest documented disease known,Tuberculosis (TB) is still a leading threat to human life. The World Health Organization (WHO) estimates that TB attacks nearly one third of the world’s population and kills about three million people each year [1]. Recent emergence of new dominant forms of TB such as multidrug resistant (MDR-TB) and extremely drug resistant (XDR-TB) are among the alarming and serious problems in TB control. Also there are a few recent cases of totally drug resistance tuberculosis (TDR),which are more dangerous,as well as a completely fatal form of tuberculosis,identified in countries like India,Iran and Italy [2, 3, 4, 5]. It is also clear from the statistical data that the mortality rate and spread of disease via TB infection in Human Immunodeficiency Virus (HⅣ) infected humans is very high compared to a normal human. Moreover,no new first line TB drug has been discovered in the last fifty years after rifampicin; except for the recently introduced drug,bedaquiline,approved by FDA for the treatment of MDR-TB. Thus,there is an urgent need for the development of newer anti-TB drugs which are more selective and effective than the existing drugs,with safe ADME (adsorption,distribution,metabolism,excretion) profiles.
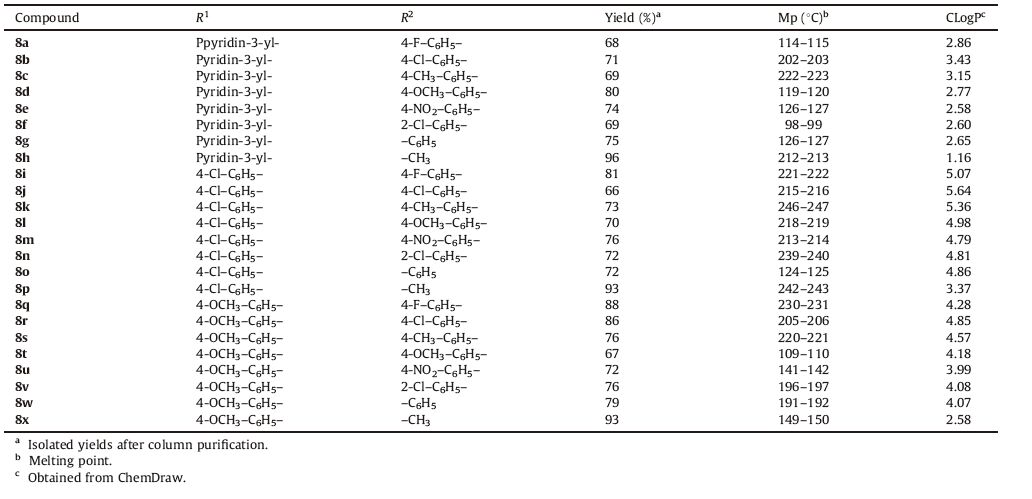
The Azole group of heterocyclic compounds is considered as prime scaffolds for the drug discovery due to their lipophilicity and the pharmacokinetic property that enhances the ability of the molecule to reach the target by transmembrane diffusion. Further,azole derivatives show promising activity against many microbials and some derivatives are also resistant to TB by inhibiting the biosynthesis of lipids [6, 7]. Furthermore,a strategy involving the amalgamation of two or more identical and different types of azoles in a single molecular framework has become the most promising route of rational drug design to yield promising pharmacophoric candidates [8, 9, 10, 11]. Among different azoles,the pyrazole derivatives attracted much attention because of their remarkable biological and pharmacological properties,such as anti-inflammatory [12, 13, 14],antimicrobial [15, 16, 17],analgesic [18],anticancer [19],etc. In recent years,several attempts were made to investigate the antitubercular activity of pyrazole derivatives. For instance,Pathak et al. [20] synthesized 3-(4-chlorophenyl)-4- substituted pyrazole derivatives and the antitubercular studies showed that most of the compounds are active against MTB; the 2- hydroxyphenyl substituted derivative (Ⅰ) (Fig. 1) is the most active with a MIC of 0.35 μg/mL. Further,Castagnolo et al. [21, 22] synthesized two series of pyrazole derivatives (Ⅱ and Ⅲ),with antitubercular activity and structure-activity relationship (SAR) studies on these analogs which revealed that the presence of the 4- chloro benzoyl moiety at C-4 of the pyrazole ring plays an important role in enhancing their activity. The 1,3,4-oxadiazole is another important member of the azole group and it is evident from the earlier literature that 1,3,4-oxadiazole derivatives exhibit a wide range of biological properties such as antimicrobial [23, 24],anticonvulsant [25],anticancer [26, 27] and,in particular,antituberculosis (such as compounds Ⅳ and V) [28, 29, 30, 31]. Therefore based on the promising antitubercular activity of these two azoles (pyrazole and 1,3,4-oxadiazole),we envisaged the molecular hybridization of these two pharmacophores as fertile research areas. A recent report [32] on the significant antitubercular activity of a series of pyrazole-oxadiazole hybrids (VI) further encouraged us in this direction. Hence,we have synthesized a new series of pyrazole-oxadiazole hybrids (8a-x) and investigated their antitubercular activity against Mycobacterium tuberculosis H37Rv (MTB) strains. Within these molecules,the 1,3,4-oxadiazole ring is attached at position-3 of the pyrazole ring,and the N1 position of which carries a phenyl ring. Another important structural feature of these molecules is the presence of amide functionality. The amide group is introduced based on the fact that the two first line TB drugs,isoniazid (INH) and pyrazinamide (PZA) contain an amide (-CONH) linkage (Fig. 1) as the major functional group. The benefit of this group is that it can easily form hydrogen bonds which increase the lipophilic character of the molecule.
|
Download:
|
| Fig. 1.Some of the active pyrazole and 1,3,4-oxadiazole based anti-TB derivatives. | |
The synthetic route for the synthesis of the final compounds 8a-x is illustrated in Scheme 1. In the first step,the methyl aryl ketone (1a-c) is condensed with diethyl oxalate to give the corresponding sodium salt of a,g-diketoesters 2a-c in good yields. These intermediates,upon cyclization with 4-nitro phenyl hydrazine in glacial acetic acid,yielded corresponding derivatives of ethyl 1-(4-nitrophenyl)-5-aryl-1H-pyrazole-3-carboxylates (3a-c). The ester derivatives were then converted to corresponding hydrazides (4a-c) by treating them with hydrazine hydrate in alcoholic media at 80 ℃ and further reaction of these hydrazides with acetic anhydride in acetic acid gave acetylated derivatives 5a-c. The cyclodehydration reaction of these intermediates at 80 ℃ using an excess of phosphorous oxychloride resulted in oxadiazole intermediates 6a-c,which upon the reduction reaction using iron powder and saturated ammonium chloride solution in 1:1 mixture of methanol and water yielded three primary amine scaffolds 7a-c.

|
Download:
|
| Scheme 1.Synthesis of pyrazole-oxadiazole hybrids (8a-x). Reagents and conditions: (a) Diethyl oxalate,NaOEt,ethanol,0 ℃ to room temperature (r.t.),24 h; (b) 4-nitro- phenyl hydrazine,HOAc,105 ℃,3 h; (c) NH2NH2.H2O,ethanol,80 ℃,3 h; (d) Ac2O,HOAc,0 ℃ to r.t.1 h; (e) POCl3,80 ℃,4 h; (f) iron powder,saturated ammonium chloride solution,methanol,70 ℃,5 h; (g) HATU,DIPEA,DMF,r.t,16 h; (h) Ac2O,HOAc,0 ℃ to r.t.,1 h. | |
The target compounds 8a-x were conveniently synthesized from the amine precursors 7a-c by using an acid-amine coupling reaction. The target derivatives (8a-g,8i-o,8q-w) were synthesized by coupling corresponding amine scaffold with different aromatic acids using the coupling agent N-[(dimethylamino)-1H- 1,2,3-triazolo-[4,5-b]pyridin-1-ylmethylene]-N-methylmethanaminium hexafluorophosphate N-oxide (HATU) and diisopropyl amine as the base in dimethyl formamide media. While the acetamide derivatives (8h,8p,8x) were synthesized by the reaction of corresponding amine scaffold with acetic anhydride in presence of acetic acid. All the final compounds (Table 1) were purified by silica gel column chromatography using 1:1 mixture petroleum ether and ethyl acetate as the eluent.
|
|
Table 1 Structural parameters,yield and melting point of the target compounds 8a-x. |
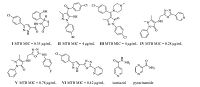
The structure of the intermediates and target compounds were confirmed by 1H NMR,13C NMR and mass spectral techniques in addition to elemental analysis. The 1H NMR spectrum of 2a displayed a triplet signal at δ 4.32 and a quartet at δ 1.33 due to methylene (-CH2-) and methyl (-CH3) protons,respectively,of the ester (-COOC2H5) group. Similarly,1H NMR spectra of 2b and 2c were in well agreement with their chemical structure. The 1H NMR spectra of compounds 3a-c show additional four protons in the aromatic region and a sharp singlet (at δ 7.34,7.18 and 7.17 for 3a,3b and 3c,respectively) due to the pyarazole ring proton which confirms the formation of 1,3,5-trisubstituted pyrazoles. The 1H NMR spectra of 4a showed two new signals at around δ 9.74 and 4.53 corresponding to -NH-and -NH2 protons,respectively,of the carbohydrazide group. Also,disappearance of triplet and quartet signals of ethyl ester protons further confirms the formation of the hydrazide intermediate. The -NH2 signal (d 4.53) of 4a was completely absent in the 1H NMR spectrum of N-acylated intermediate 5a,whereas two new signals appeared at δ 10.18 and 1.91 due to -NH and -CH3 groups,respectively. Also,in the 13C NMR spectra of 5a,a new peak was observed at δ 20.04 confirming the presence of -CH3 group. Similar signals in the 1H and 13C NMR spectra of 5b and 5c confirms their structures. The successful cyclization of 5a-c to form oxadiazole derivatives 6a-c was established by both 1H NMR and 13C NMR data. For instance,absence of both -NH-signals and shift of -CH3 signal from δ 1.91 to 2.61 in the 1H NMR spectrum and also shift of -CH3 signal from d 20.04 to 11.04 in the 13C NMR spectrum of 6a supports the successful cyclization of 5a. The synthesis of intermediate 7a by the reduction of nitro group in 6a to amine group was well supported by the appearance of a new broad singlet for -NH2 group at δ 5.50 in their 1H NMR spectra. The structure of target compounds,N-(4-(5-aryl-3-(5-methyl-1,3,4-oxadiazol-2-yl)-1Hpyrazol- 1-yl)phenyl)-4-amides,was well confirmed by spectral methods and the characterization data are given in the electronic Supporting information. For instance,successful acid-amine coupling to form an amide group in the target compound 8a is evident by the disappearance of -NH2 signal and appearance of new amide -NH-signal at δ 10.51 in its 1H NMR spectrum. Further,the spectrum showed the presence of four additional protons in the aromatic region due to the introduction of a new phenyl ring. The 13C NMR spectrum of 8a matches well with its structure. The mass spectrum of 8a showed a molecular ion peak [M + H] at m/z 441.2,which corresponds to its molecular formula C24H17FN6O2 (MW = 440.43). Similarly,the formation of acetamide derivatives (8h,8p and 8x) was confirmed by both 1H and 13CNMR spectra. For instance,formation of N-(4-(3-(5-methyl-1,3,4-oxadiazol-2-yl)-5- (pyridin-3-yl)-1H-pyrazol-1-yl)phenyl)acetamide (8h) was supported by the appearance additional -CH3 signals at δ 2.05 and d 24.52 in its 1H NMR and 13C NMR spectra respectively,whereas the -NH2 signal was completely absent in the 1H NMR spectrum. Further,the structure one of the final derivatives (8p) was achieved by SC-XRD analysis and the ORTEP diagram for the compound is shown in Fig. 2.
|
Download:
|
| Fig. 2.The ORTEP diagram showing the X-ray crystal structure of 8p. | |
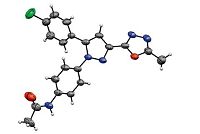
The in vitro antimycobacterial activity of the newly synthesized compounds 8a-x was evaluated by screening them against M. tuberculosis H37Rv (ATCC27294) by agar dilution method. The experiment was carried out in triplicates and the minimum inhibitory concentration (MIC) was determined. The MIC is defined as the minimum concentration of compound required to completely inhibit the bacterial growth. The comparison of the MIC values (in μg/mL) of 8a-x with the standard drugs is shown in Fig. 3. The compound,2-chloro-N-(4-(5-(4-chlorophenyl)-3-(5- methyl-1,3,4-oxadiazol-2-yl)-1H-pyrazol-1-yl)phenyl)benzamide (8n) is the lead molecule of the series with MIC of 1.56 μg/mL and is more potent than the standard,ethambutol. Whereas,the unsubstituted benzamide derivative 8o is the next most active molecule with MIC of 3.13 μg/mL,which is comparable with ethambutol. The derivatives 8e,8l and 8x showed promising antitubercular activity with MIC of 6.25 μg/mL while moderate inhibition was observed with compounds 8m,8s,8t and 8u (MIC = 12.5 μg/mL). It is evident from the MIC values that the antitubercular activity varies with different substitution (R1) at position-5 of the pyrazole ring. Further,the activity also depends on nature and position of the substituent on the phenyl ring of the benzamide moiety (see R2,Table 1). Among compounds derived from three different scaffolds (7a-c),most analogs of 4-chloro phenyl substituted pyrazole scaffold (7b) showed better antitubercular activity when compared with the activity of those derived from pyridin-3-yl-(7a) and 4-methoxy phenyl substituted pyrazole (7c) scaffolds. Except the nitro derivative 8e,no other pyridine derivatives showed promising activity. In the case of 4-chloro phenyl substituted pyrazoles,it is interesting to know that though 2-chloro benzamide derivative (8n) is significantly active (MIC = 1.56 μg/mL) corresponding 4-chloro derivative did not show a prominent activity (MIC = 50 μg/mL). This observation indicates that variation in the position (ortho or para) of the chloro group largely effects the activity of these molecules,the exact reason for this effect may be explored by further studies on the structure-activity relationship,or by computer aided modeling studies. The promising activity of 4-chloro phenyl substituted pyrazoles prompted us to investigate the activity of corresponding intermediates 4b,5b,6b and 7b. Among the four compounds,5b showed significant activity with MIC of 3.13 μg/mL (Fig. 3) while the other three compounds were not so active (MIC = 50 μg/mL). It is evident that the replacement of the N-acylcarbohydrazide (-CONHNHCOCH3) functionality in 5b with either a carbohydrazide (-CONHNH2) group (4b) or an oxadiazole group (6b) reduced the activity of the molecules. This observation signifies the contribution of the acylcarbohydrazide functionality towards the antitubercular activity. Thus,compound 5b could be considered as a lead molecule for further structural modifications and biological studies in order to develop efficient antitubercular drugs.

|
Download:
|
| Fig. 3.The antitubercular activity of pyrazole-1,3,4-oxadiazole derivatives (8a-x),and intermediates 4b,5b,6b and 7b. | |
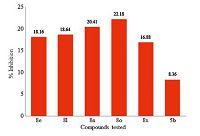
The in vitro cytotoxicity of the activemolecules (MIC × 6.25 μg/mL) was assessed by MTT assay against NIH 3T3 cells at 50 μg/mL concentration. The compounds are non-toxic at the tested concentration with cell growth inhibition values in the range 16.88%-22.18% (Fig. 4). Interestingly,the intermediate 5b showed the least toxicity (8.36% inhibition).
|
Download:
|
| Fig. 4.The cytotoxicity of the active derivatives against NIH 3T3 cells at 50 μg/mL concentration. | |
In this study,we demonstrated the synthesis and antitubercular study of a new series of N-(4-(5-aryl-3-(5-methyl-1,3,4-oxadiazol- 2-yl)-1H-pyrazol-1-yl)phenyl)-4-amide analogs. Nine compounds showed promising antitubercular activity against MTB strain with MIC × 12.5 μg/mL among which 2-chloro-N-(4-(5-(4-chlorophenyl)- 3-(5-methyl-1,3,4-oxadiazol-2-yl)-1H-pyrazol-1-yl)phenyl)- benzamide (8n) was the most active molecule with a MIC of 1.56 μg/mL. The structure and activity relationship revealed that the 4-chloro phenyl substituted pyrazole derivatives should be more effective antitubercular agents than the corresponding pyridine and 4-methoxy phenyl substituted derivatives. Among the 4-chloro phenyl substituted pyrazole intermediates (4b-7b),compound 5b containing an N-acylcarbohydrazide group showed significant activity (MIC = 3.13 μg/mL). The cytotoxicity studies further establish that the active molecules of the series are nontoxic to a normal cell line and hence are potential lead molecules for the drug development process.
AcknowledgmentsThe authors are thankful to National Institute of Technology Karnataka,India for the financial support and laboratory facility. We thank MIT Manipal and Dr. Reddy’s Institute of Life Sciences,University of Hyderabad Campus,Hyderabad for NMR and mass spectral facility. The authors are thankful to Maratha Mandal’s NGH Institute of Dental Sciences & Research Centre,Belgaum for the cytotoxicity studies.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2016.01.015.
| [1] | World Health Organization, Global Tuberculosis Report 2014, (accessed April 2015). |
| [2] | L. Ballell, R.A. Field, K. Duncan, R.J. Young, New small-molecule synthetic antimycobacterials, Antimicrob. Agents Chemother. 49(2005) 2153-2163. |
| [3] | A.A. Velayati, M.R. Masjedi, P. Farnia, et al., Emergence of new forms of totally drug-resistant tuberculosis bacillisuper extensively drug-resistant tuberculosis or totally drug-resistant strains in (Ⅰ)ran, Chest J. 136(2009) 420-425. |
| [4] | S. Loewenberg, (Ⅰ)ndia reports cases of totally drug-resistant tuberculosis, Lancet 379(2012) 205. |
| [5] | A.S. Fauci, Multidrug-resistant and extensively drug-resistant tuberculosis:the national institute of allergy and infectious diseases research agenda and recommendations for priority research, J. (Ⅰ)nfect. Dis. 197(2008) 1493-1498. |
| [6] | A. Andreani, M. Granaiola, A. Leoni, et al., Synthesis and antitubercular activity of imidazo[21-b]thiazoles, Eur. J. Med. Chem. 36(2001) 743-746. |
| [7] | S.G. Kini, A.R. Bhat, B. Bryant, J.S. Williamson, F.E. Dayan, Synthesis, antitubercular activity and docking study of novel cyclic azole substituted diphenyl ether derivatives, Eur. J. Med. Chem. 44(2009) 492-500. |
| [8] | Y. Sheng, B. Sun, X. Xie, N. Li, D. Dong, DMH1(4-[6-(4-isopropoxyphenyl) pyrazolo[1,5-a]pyrimidin-3-yl]quinoline) inhibits chemotherapeutic drug-induced autophagy, Acta. Pharm. Sin., B 5(2015) 330-336. |
| [9] | C. Han, Y.C. Guo, D.D. Wang, et al., Novel pyrazole fused heterocyclic ligands:synthesis, characterization. DNA binding/cleavage activity and anti-BVDV activity, Chin. Chem. Lett. 26(2015) 534-538. |
| [10] | S.L. Wang, Y.J. Shi, H.B. He, et al., Synthesis and bioactivity of novel pyrazole oxime derivatives containing oxazole ring, Chin. Chem. Lett. 26(2015) 672-674. |
| [11] | R.K. Arora, N. Kaur, Y. Bansal, G. Bansal, Novel coumarin-benzimidazole derivatives as antioxidants and safer anti-inflammatory agents, Acta. Pharm. Sin., B 4(2014) 368-375. |
| [12] | N. Chandna, J.K. Kapoor, J. Grover, et al., Pyrazolylbenzyltriazoles as cyclooxygenase inhibitors:synthesis and biological evaluation as dual anti-inflammatory and antimicrobial agents, New J. Chem. 38(2014) 3662-3672. |
| [13] | S.G. Alegaon, K.R. Alagawadi, M.K. Garg, K. Dushyant, D. Vinod, 1,3,4-Trisubstituted pyrazole analogues as promising anti-inflammatory agents, Bioorg. Chem. 54(2014) 51-59. |
| [14] | B.V. Kendre, M.G. Landge, W.N. Jadhav, S.R. Bhusare, Synthesis and bioactivities of some new 1H-pyrazole derivatives containing an aryl sulfonate moiety, Chin. Chem. Lett. 24(2013) 325-328. |
| [15] | A. Tanitame, Y. Oyamada, K. Ofuji, et al., Synthesis and antibacterial activity of a novel series of potent DNA gyrase inhibitors. Pyrazole derivatives, J. Med. Chem. 47(2004) 3693-3696. |
| [16] | G.M. Nitulescu, C. Draghici, M.C. Chifiriuc, et al., Synthesis and antimicrobial screening of N-(1-methyl-1H-pyrazole-4-carbonyl)-thiourea derivatives, Med. Chem. Res. 21(2012) 308-314. |
| [17] | H.H. Jardosh, C.B. Sangani, M.P. Patel, R.G. Patel, One step synthesis of pyrido[1,2-a]benzimidazole derivatives of aryloxypyrazole and their antimicrobial evaluation, Chin. Chem. Lett. 24(2013) 123-126. |
| [18] | A. Hall, A. Billinton, S.H. Brown, et al., Non-acidic pyrazole EP 1 receptor antagonists with in vivo analgesic efficacy, Bioorg. Med. Chem. Lett. 18(2008) 3392-3399. |
| [19] | K.M. Dawood, T.M. Eldebss, H.S. El-Zahabi, M.H. Yousef, P. Metz, Synthesis of some new pyrazole-based 1,3-thiazoles and 1,3,4-thiadiazoles as anticancer agents, Eur. J. Med. Chem. 70(2013) 740-749. |
| [20] | R.B. Pathak, P.T. Chovatia, H.H. Parekh, Synthesis, antitubercular and antimicrobial evaluation of 3-(4-chlorophenyl)-4-substituted pyrazole derivatives, Bioorg. Med. Chem. Lett. 22(2012) 5129-5133. |
| [21] | D. Castagnolo, A. De Logu, M. Radi, et al., Synthesis, biological evaluation and SAR study of novel pyrazole analogues as inhibitors of Mycobacterium tuberculosis, Bioorg. Med. Chem. 16(2008) 8587-8591. |
| [22] | D. Castagnolo, F. Manetti, M. Radi, et al., Synthesis, biological evaluation, and SAR study of novel pyrazole analogues as inhibitors of Mycobacterium tuberculosis:Part 2. Synthesis of rigid pyrazolones, Bioorg. Med. Chem. 17(2009) 5716-5721. |
| [23] | H. Chen, Z. Li, Y. Han, Synthesis and fungicidal activity against Rhizoctonia solani of 2-alkyl (alkylthio)-5-pyrazolyl-1,3,4-oxadiazoles (thiadiazoles), J. Agric. Food Chem. 48(2000) 5312-5315. |
| [24] | G. Ş ahin, E. Palaska, M. Ekizoğlu, M.Özalp, Synthesis and antimicrobial activity of some 1,3,4-oxadiazole derivatives, (Ⅰ)l Farmaco 57(2002) 539-542. |
| [25] | A. Zarghi, S.A. Tabatabai, M. Faizi, et al., Synthesis and anticonvulsant activity of new 2-substituted-5-(2-benzyloxyphenyl)-13,4-oxadiazoles, Bioorg. Med. Chem. Lett. 15(2005) 1863-1865. |
| [26] | S. Valente, D. Trisciuoglio, T. De Luca, et al., 1,3,4-Oxadiazole-containing histone deacetylase inhibitors:anticancer activities in cancer cells, J. Med. Chem. 57(2014) 6259-6265. |
| [27] | P. Miralinaghi, M. Salimi, A. Amirhamzeh, et al., Synthesis, molecular docking study, and anticancer activity of triaryl-1, 2,4-oxadiazole, Med. Chem. Res. 22(2013) 4253-4262. |
| [28] | M.J. Ahsan, J.G. Samy, H. Khalilullah, et al., Molecular properties prediction and synthesis of novel 1,3,4-oxadiazole analogues as potent antimicrobial and antitubercular agents, Bioorg. Med. Chem. Lett. 21(2011) 7246-7250. |
| [29] | M.J. Ahsan, J.G. Samy, C.B. Jain, et al., Discovery ofnovel antitubercular15-dimethyl-2-phenyl-4-([5-(arylamino)-1,3,4-oxadiazol-2-yl] methylamino)-1,2-dihydro-3Hpyrazol-3-one analogues, Bioorg. Med. Chem. Lett. 22(2012) 969-972. |
| [30] | R.A. Rane, S.D. Gutte, N.U. Sahu, Synthesis and evaluation of novel 1,3,4-oxadiazole derivatives of marine bromopyrrole alkaloids as antimicrobial agent, Bioorg. Med. Chem. Lett. 22(2012) 6429-6432. |
| [31] | R.A. Rane, P. Bangalore, S.D. Borhade, P.K. Khandare, Synthesis and evaluation of novel 4-nitropyrrole-based 1,3,4-oxadiazole derivatives as antimicrobial and anti-tubercular agents, Eur. J. Med. Chem. 70(2013) 49-58. |
| [32] | P. Horrocks, M. Pickard, H. Parekh, S. Patel, R.B. Pathak, Synthesis and biological evaluation of 3-(4-chlorophenyl)-4-substituted pyrazole derivatives, Org. Biomol. Chem. 11(2013) 4891-4898. |
 2016, Vol.27
2016, Vol.27