b. Nano and Biotechnology Research Group, University of Mazandaran, Babolsar 47416-95447, Iran;
c. Department of Biology, Garmsar Branch, Islamic Azad University, Garmsar 47416-56666, Iran
Bridgehead nitrogenheterocycles play a prominent role in nature due to their presence as a key scaffold in biologically active pharmaceuticals,agrochemicals,and functional materials [1, 2]. Among this class of heterocyclic compounds,indolizine derivatives have attracted considerable attention with respect to their significant biological activities,including antioxidant [3],antimicrobial [4],anti-inflammatory [5],analgesic [6],antiviral [7],antitumor [8],hypoglycemic [9],CNS depressant [10],antiacetylcholine [11],and 5-HT3 receptor antagonists activities [12]. In addition,some indolizine compounds have been found to possess fluorescence activities [13] and the capacity to formsurface films [14],which enables them for designing novel classes of fluorescent dyes [15],chemosensors [16],and biological markers [17]. Owing to the increasing importance of indolizine derivatives,new and efficient methods have been offered for the synthesis of these heterocycles by synthetic organic chemists [18, 19].
The reactions of nitrogen heterocycles like pyridine and isoquinoline with activated acetylenes proceeded through a zwitterionic intermediate [20, 21],which undergoes further reaction with electron-deficient carbonyl groups such as ninhydrin [22],ethyl pyruvate [23],benzofuran-2,3-diones [24],1,3- dimethylalloxan [25],to afford spiro[1, 3]-oxazines. However,when a-halogen substituted ketones were used as electrophiles in these reactions,different results were reported. Pyridine and 3- substituted pyridines (X = CH3,Cl,Br) in reaction with mono-and di-a-halo ketones and DMAD led to formation of the corresponding [1, 3]-oxazines [26]. In addition,pyridine in reaction with hexachloroacetone [27] under the same reaction conditions and phenacyl bromide [28] in the presence of basic alumina using microwave energy,resulted in indolizine derivatives. These results encouraged us to study the reactions of amino pyridines with ahalo ketones and dialkyl acetylenedicarboxylates in more detail.
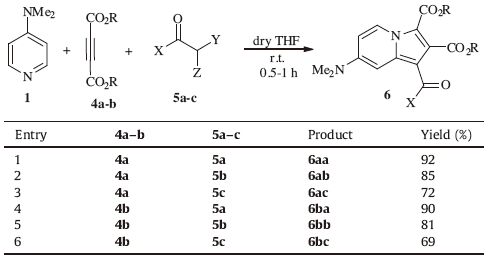
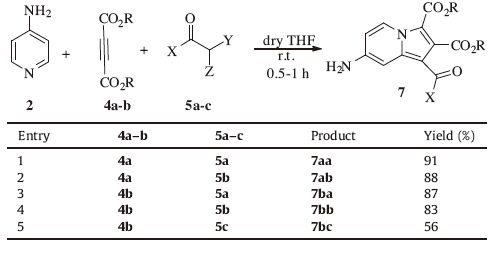
Herein,we report three-component reactions of pyridines 1-3 and dialkyl acetylenedicarboxylates 4a-c with different a-halo ketones 5a-c in dichloromethane at room temperature resulting in chemoselective synthesis of indolizine derivatives 6-8 without formation of any [1, 3]-oxazines 9 (Scheme 1).

|
Download:
|
| Scheme 1.Synthesis of indolizine derivatives 6-8. | |
Dimethyl acetylenedicarboxylate (DMAD),diethyl acetylenedicarboxylate (DEAD),di-tert-butylacetylene-dicarboxylate (DTAD),3-aminopyridine,4-aminopyridine,4-dimethylaminopyridine (DMAP),2,2-dichloroacetophenone (=2,2-dichloro-1-phenylethanone),1,1-dichloroacetone (=1,1-dichloropropan-2-one),ethyl bromopyruvate,phenacyl chloride,phenacyl bromide,and chloroacetone were purchased from Fluka and Merck and used without further purification. Melting points were measured on an Electrothermal 9100 apparatus. NMR spectra were recorded with a Bruker DRX-400 AVANCE instrument (400.1 MHz for 1H,100.6 MHz for 13C) with CDCl3 and DMSO-d6 as solvents.
Chemical shifts are given in parts per million (d) relative to TMS,and coupling constants (J) are reported in hertz (Hz). IR spectra were recorded on an FT-IR Bruker vector 22 spectrometer. Mass spectra were recorded on a Finnigan-Matt 8430 mass spectrometer operating at an ionization potential of 70 eV. Elemental analyses were carried with a Perkin- Elmer 2400II CHN Elemental Analyzer.
2.1. General procedure for synthesis of compounds 6aa-8cbTo a magnetically stirred solution of a-halo ketones (1 mmol) and acetylenic diesters (1 mmol) in dry THF (10 mL) at ambient temperature was added dropwise a solution of aminopyridines (1 mmol) in dry THF (2 mL) over 10 min. The reaction mixture was then allowed to stir for 0.5-1 h. After completion of the reaction,as indicated by TLC (n-hexane/ethyl acetate,7:3),the solvent was removed under reduced pressure. The residue was purified by column chromatography on silica gel (Merck,230-240 mesh) using a mixture of n-hexane/ethyl acetate (3:2) as eluent to give the desired product.
Dimethyl 1-benzoyl-7-(dimethylamino)indolizine-2,3-dicarboxylate (6aa): Yellow powder,mp: 212-214 ℃; yield (0.35 g,92%); IR (KBr,cm-1): vmax 3061 (Csp 2-H),2995 and 2948 (Csp 3-H),1738 (C=O,ester),1689 (C=O,ketone),1647 (C=C),1230 (Csp 2-O),1098 (Csp 3-O); 1H NMR (400.1 MHz,CDCl3): δ 3.05 (s,6H,NMe2),3.51 and 3.86 (2s,6H,2 × OMe),6.66 (dd,1H,3JHH = 7.6 Hz,4JHH = 2.4 Hz,CH),7.10 (d,1H,4JHH = 2.4 Hz,CH),7.42-7.52 (m,3H,2CHortho and CHpara),7.64-7.67 (m,2H,2CHmeta),9.31 (d,1H,3JHH = 7.6 Hz,CH); 13C NMR (100.6 MHz,CDCl3): δ 39.8 (NMe2),51.7 and 52.3 (2 × OMe),96.1 and 105.3 (2 × CH),108.8 and 110.4 (2Cq),128.0 (2CHortho),128.2 (2CHmeta),128.7 (CH),130.8 (CHpara),131.4 and 141.1 (2Cq),141.8 (Cipso),149.1 (CNMe2),160.9 and 166.2 (2C=O,ester),190.4 (C=O,ketone); MS,m/z (%): 380 (M+•,100),349 (12),303 (=),277 (16),262 (8),245 (16),219 (7),186 (8),159 (11),139 (6),105 (8),77 (18); Anal. Calcd. for C21H20N2O5 (380.39): C 66.31,H 5.30,N 7.36%. Found: C 66.51,H 5.32,N 7.38%.
Experimental procedures and spectral data of new compounds are available in Supporting information file.
3. Results and discussionInitially,three component reaction of 4-N,N-dimethylaminopyridine (DMAP) 1 with DMAD 4a in the presence of 5a was carried out at room temperature for 0.5 h affording the corresponding indolizine 6aa in high yield (Table 1,entry 1). When this reaction was performed with a-bromopyrovate 5c,the yield of the indolizine product 6ac significantly decreased (Table 1,entry 3). This could be due to lower electrophilicity of the Ca relative to the carbonyl group in compound 5c. DMAP 1 reacted smoothly with dialkyl acetylenedicarboxylate 4b and ketones 5a-c to form the indolizines 6ba-6bc (Table 1,entries 4-6). Similarly,4-aminopyridine 2 and 3-aminopyridine 3 treated with acetylenic esters 4a-c and ketones 5a-c under the same reaction conditions and moderate to high yields of expected indolizine products were obtained and results are shown in Tables 2 and 3,respectively. Lower yields of the products in the case of 3-aminopyridine 3 (Table 3) relative to results of DMAP 1 and 4-minopyridine 2 (Tables 1 and 2) might be due to lower nucleophilicity of the nitrogen atom of pyridine in 3-aminopyridine 3.
|
|
Table 1 Synthesis of indolizine derivatives 6aa-6bc using 4-N,N-dimethyl aminopyridine. |
|
|
Table 2 Synthesis of indolizine derivatives 7aa-7bc using 4-aminopyridine. |
|
|
Table 3 Synthesis of indolizine derivatives 8aa-8cb using 3-aminopyridine. |
To extend further the scope of this reaction,pyridines 1-3 reacted with DMAD 4a and mono a-halo ketones 10,such as phenacyl chloride,phenacyl bromide,and chloroacetone,under the same reaction conditions. Only two component products 11 were formed without formation of any indolizines 12,and unreacted ketones 10 were recovered (Scheme 2). These results further confirm that electrophilicity of Ca is important and seems essential for the reaction to proceed.

|
Download:
|
| Scheme 2.Reaction of aminopyridines with mono a-halo ketones. | |
The structures of compounds 6aa-8cb were characterized on the basis of their IR,1H NMR,and 13C NMR spectroscopic methods,mass spectrometry,and elemental analysis. For example,the IR spectrum of 6aa displayed absorption bands related to the carbonyl groups of esters at 1738 cm-1,the carbonyl group of ketone at 1689 cm-1,C=C group at 1647 cm-1,and the absorption bands of C-O stretching at 1230 and 1098 cm-1. In the 1H NMR spectrum of 6aa,the dimethylamino protons were observed at 3.05 ppm as a singlet,along with two singlets at 3.51 and 3.86 ppm for the two methoxy protons,a doublet of doublet at 6.66 ppm (3JHH = 7.6 Hz,4JHH = 2.4 Hz),a doublet at 7.10 ppm (4JHH = 2.4 Hz) and a doublet at 9.31 ppm (3JHH = 7.6 Hz) for the three CH groups of the pyridine ring. The signals due to the phenyl ring moiety were discernible as two characteristic multiplets in the appropriate regions (7.42-7.52 and 7.64-7.67 ppm). The 13C NMR spectrum of 6aa exhibited eighteen signals in accordance with the suggested structure. The characteristic signals of the carbonyl groups of esters and ketone were observed at 160.9,166.2 and 190.4 ppm,respectively. The mass spectrum of 6aa showed molecular ion signal at m/z 380,which is consistent with the 1:1:1 adduct of 4- dimethylaminopyridine,DMAD,and 2,2-dichloroacetophenone.
The 1H NMR and 13C NMR spectra of 6ab-8cb were similar to those of 6aa except for the ester alkoxy groups and substituents on the pyridine and ketone moieties which exhibited characteristic signalswith appropriate chemical shifts and coupling constants. The IR spectra of compounds 6ab-8cb were in agreement with the proposed structures. In the case of compounds 7aa-8cb,the IR spectrum showed two absorption bands at the range of 3465 and 3314 cm-1 for theNH2 group. Themass spectra of 6ab-8cb exhibited the molecular ion peaks at the appropriate m/z values. Initial fragmentations involved loss of side chains of indolizine moiety.
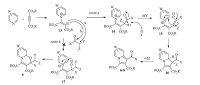
A plausible mechanism for this transformation is proposed in Scheme 3. On the basis of the chemistry of N-heterocycle nucleophiles [29, 30, 31],it is assumed that the zwitterionic intermediate 13 results from an initial addition of aminopyridine to the dialkyl acetylenedicarboxylate [26, 27]. Attack of intermediate 13 at the halogenated carbon atom (Cα) of the α-halo ketone generates the salt 14,which subsequently loses a proton to form 15. The zwitterionic intermediate 15 undergoes cyclization to give 16,which eliminates HZ to afford indolizines 6-8 (route a). Alternatively,the intermediate 13 can attack the C=O group of the α-halo ketone,leading to the dipolar species 17,which then undergoes cyclization to generate [1, 3]-oxazines 9 (route b). Since the 13C NMR spectra of the products exhibited a signal at the carbonyl region (~200 ppm),route a is suggested as a tentative mechanism for this transformation.
|
Download:
|
| Scheme 3.Proposed mechanism for the synthesis of compounds 6-8. | |
In conclusion,we have described three-component reactions of aminopyridines and dialkyl acetylenedicar-boxylates with activated a-halo ketones to produce novel aminoindolizine derivatives. The use of simple starting materials,high yield of products,mild conditions,and operational simplicity are the salient advantages of the present procedure. Furthermore,valuable fluorescence properties were reported for some of the synthesized indolizines in this work [32]. Also,the obtained functionalized indolizine derivatives can be used as key intermediates for synthesis of new organic compounds.
AcknowledgmentThis research was supported by the Research Council of the University of Mazandaran,Iran.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.11.007.
| [1] | F.J. Swinbourne, J.H. Hunt, G. Klinkert, Advances in indolizine chemistry, Adv. Heterocycl. Chem. 23(1979) 103-170. |
| [2] | (Ⅰ). Hermecz, L. Vasvári-Debreczy, P. Mátyus, Bicyclic 6-6 systems with one ring junction nitrogen atom:one extra heteroatom 1:0, in:A.R. Katritzky, C.W. Rees, E.F.V. Scriven (Eds.), Comprehensive Heterocyclic Chemistry Ⅱ, Pergamon Press, London, 1996, pp. 563-595. |
| [3] | A.(Ⅰ). Nasir, L.L. Gundersen, F. Rise, et al., (Ⅰ)nhibition of lipid peroxidation mediated by indolizines, Bioorg. Med. Chem. Lett. 8(1998) 1829-1832. |
| [4] | L.L. Gundersen, C. Charnock, A.H. Negussie, F. Rise, S. Teklu, Synthesis of indolizine derivatives with selective antibacterial activity against Mycobacterium tuberculosis, Eur. J. Pharm. Sci. 30(2007) 26-35. |
| [5] | H. Malonne, J. Hanuise, J. Fontaine, Topical anti-inflammatory activity of new 2-(1-indolizinyl)propionic acid derivatives in mice, Pharm. Pharmacol. Commun. 4(1998) 241-243. |
| [6] | F. Campagna, A. Carotti, G. Casini, M. Macripò, Synthesis of new heterocyclic ring systems:indeno[2,1-b]-benzo[g] indolizine and indeno[1', 2':5, 4] pyrrolo[2,1-a] phthalazine, Heterocycles 31(1990) 97-107. |
| [7] | S. Medda, P. Jaisankar, R.K. Manna, et al., Phospholipid microspheres:a novel delivery mode for targeting antileishmanial agent in experimental leishmaniasis, J. Drug Target. 11(2003) 123-128. |
| [8] | K. Olden, P. Breton, K. Grzegorzewski, et al., The potential importance of swainsonine in therapy for cancers and immunology, Pharmacol. Ther. 50(1991) 285-290. |
| [9] | A.U. De, B.P. Saha, Search for potential oral hypoglycemic agents, J. Pharm. Sci. 64(1975) 249-252. |
| [10] | W.B. Harrell, R.F. Doerge, New compounds:Mannich bases from 2-phenylindolizines Ⅱ. 3-Dialkylaminomethyl derivatives, J. Pharm. Sci. 56(1967) 1200-1202. |
| [11] | (Ⅰ). Antonini, F. Claudi, U. Gulini, L. Micossi, F. Venturi, (Ⅰ)ndolizine derivatives with biological activity (Ⅰ)V:3-(2-Aminoethyl)-2-methylindolizine, 3-(2-aminoethyl)-2-methyl-5,6,7,8-tetrahydroindolizine, and their iv-alkyl derivatives, J. Pharm. Sci. 68(1979) 321-324. |
| [12] | J. Bermudez, C.S. Fake, G.F. Joiner, et al., 5-Hydroxytryptamine (5-HT3) receptor antagonists. 1. (Ⅰ)ndazole and indolizine-3-carboxylic acid derivatives, J. Med. Chem. 33(1990) 1924-1929. |
| [13] | A. Vlahovici, (Ⅰ). Druă, M. Andrei, M. Cotlet, R. Dinică, Photophysics of some indolizines, derivatives from bipyridyl, in various media, J. Lumin. 82(1999) 155-162. |
| [14] | J.B. Henry, R.J. MacDonald, H.S. Gibbad, H. McNab, A.R. Mount, The formation and characterisation of redox active and luminescent materials from the electrooxidation of indolizine, Phys. Chem. Chem. Phys. 13(2011) 5235-5241. |
| [15] | A.V. Rotaru, (Ⅰ).D. Druta, T. Oeser, T.J. Müller, A novel coupling 1,3-dipolar cycloaddition sequence as a three-component approach to highly fluorescent indolizines, Helv. Chim. Acta 88(2005) 1798-1812. |
| [16] | M. Becuwe, F. Delattre, G.G. Surpateanu, et al., Synthesis of new fluorescent β-cyclodextrin sensor, Heterocycl. Commun. 11(2005) 355-360. |
| [17] | F. Delattre, P. Woisel, G. Surpateanu, F. Cazier, P. Blach, 1-(4-Nitrophenoxycarbonyl)-7-pyridin-4-yl indolizine:a new versatile fluorescent building block. Application to the synthesis of a series of fluorescent β-cyclodextrins, Tetrahedron 61(2005) 3939-3945. |
| [18] | R. Bonneau, Y.N. Romashin, M.T.H. Liu, S.E. MacPherson, Synthesis of 3-substituted indolizines from the reaction of chlorocarbenes with 2-vinylpyridine, J. Chem. Soc., Chem. Commun. 4(1994) 509-510. |
| [19] | D.A. Goff, Combinatorial synthesis of indolizines on solid support, Tetrahedron Lett. 40(1999) 8741-8745. |
| [20] | V. Nair, A.R. Sreekanth, N. Abhilash, et al., Novel pyridine-catalyzed reaction of dimethyl acetylenedicarboxylate with aldehydes and N-tosylimines:efficient synthesis of 2-benzoylfumarates and 1-azadienes, Synthesis 2003(2003) 1895-1902. |
| [21] | V. Nair, A.R. Sreekanth, A.U. Vinod, Novel pyridine-catalyzed reaction of dimethyl acetylenedicarboxylate with aldehydes:formal[2+2] cycloaddition leading to 2-oxo-3-benzylidinesuccinates, Org. Lett. 3(2001) 3495-3497. |
| [22] | (Ⅰ). Yavari, Z. Hossaini, M. Sabbaghan, M. Ghazanfarpour-Darjani, Reaction of Nheterocycles with acetylenedicarboxylates in the presence of N-alkylisatins or ninhydrin. Efficient synthesis of spiro compounds, Monatsh. Chem. 138(2007) 677-681. |
| [23] | (Ⅰ). Yavari, A. Mirzaei, Z. Hossaini, S. Souri, Diastereoselective synthesis of fused[1,3] oxazines from ethyl pyruvate, activated acetylenes and N-heterocycles, Mol. Diversity 14(2010) 343-347. |
| [24] | A.A. Esmaeili, H. Vesalipoor, R. Hosseinabadi, et al., An efficient diastereoselective synthesis of spiro pyrido[2,1-b][1,3] oxazines via a novel pyridine-based threecomponent reaction, Tetrahedron Lett. 52(2011) 4865-4867. |
| [25] | M.B. Teimouri, T. Abbasi, S. Ahmadian, M.R. Poor Heravi, R. Bazhrang, An efficient three-component protocol for the synthesis of novel spiro-oxazinobarbiturates, Tetrahedron 65(2009) 8120-8124. |
| [26] | S. Asghari, A.K. Habibi, One pot three-component regioselective and diastereoselective synthesis of halogenated pyrido[2,1-b][1,3] oxazines, Tetrahedron 68(2012) 8890-8898. |
| [27] | (Ⅰ). Yavari, M. Sabbaghan, Z. Hossaini, Reaction of hexachloroacetone with activated acetylenes in the presence of N-heterocycles. Synthesis of trichloremethylated bridgehead N-heterocycles, Synlett 15(2006) 2501-2503. |
| [28] | U. Bora, A. Saikia, R.C. Boruah, A novel microwave-mediated one-pot synthesis of indolizines via a three-component reaction, Org. Lett. 5(2003) 435-438. |
| [29] | O. Diels, K. Alder, Synthesen in der hydroaromatischen Reihe. XVⅡ. Mitteilung. "Dien-Synthesen" stickstoffhaltiger Heteroringe. 5. Dien-Synthesen des Pyridins, Chinolins, Chinaldins und (Ⅰ)sochinolins, Justus Liebigs, Ann. Chem. 498(1932) 16-49. |
| [30] | (Ⅰ). Yavari, N. Hosseini, L. Moradi, An efficient synthesis of 2-cyano-2-phenyl-2, 11b-dihydro-[1,3] oxazino[2,3-a] isoquinolines by reaction of isoquinoline with electron-deficient acetylenes in the presence of benzoylcyanide, Monatsh. Chem. 139(2008) 953-956. |
| [31] | R. Huisgen, M. Morikawa, K. Herbig, E. Brunn, 1.4-Dipolare cycloadditionen, Ⅱ. Dreikomponenten-Reaktionen des (Ⅰ)sochinolins mit Acetylendicarbonsäureester und verschiedenen Dipolarophilen, Chem. Ber. 100(1967) 1094-1106. |
| [32] | M.J. Chaichi, M. Ehsani, S. Asghari, V. Behboodi, Determination of vitamin B6 using an optimized novel TCPO-indolizine-H2O2 chemiluminescence system, Luminescence 29(2014) 1169-1176. |
 2016, Vol.27
2016, Vol.27