b. Chemistry Department, Faculty of Science, Mansoura University, Mansoura 35516, Egypt
Bis(indolyl)alkanes have important biological,industrial,and synthetic applications. Thus,their preparation is of considerable interest for the researchers in the development of new protocols. In recent years,syntheses of this class of molecules under mild conditions have been reported,with promoters such as Montmorillonite clay K-10 [1] in solid state reactions [2],trichloro-1,3,5- triazine [3],AlPW12O40 [4],sodium dodecyl sulfate (SDS) [5],ZrCl4 [6],I2 [7],In(OTf)3/ionic liquid [8],CuBr2 [9],MW/Lewis acids (FeCl3,BiCl3,InCl3,ZnCl2,CoCl2) [10],NaHSO4 and Amberlyst-15 [11],silica sulfuric acid (SSA) [12],metal hydrogen sulfates [13], NaHSO4/ionic liquid [14],CAN [15],NBS [16],and Ph3CCl [17].
Amides and bisamides are functionalized groups represent important biological and medicinal scaffolds,which play a major role in the development and composition of biological and pharmacological systems [18, 19]. In particular,symmetrical and unsymmetrical N,N 0-alkylidene bisamides and their derivatives are found as key structural subunits for the construction of peptidomimetic frameworks [20, 21]. Recently,Perumal et al. [22] have reported an alternative approach to synthesize symmetrical N,N 0 - alkylidene bisamides by a reaction of aldehydes with nitriles in the presence of sulfamic acid. However,the yields were moderate. Milenkovic et al. [23] have synthesized activated imines and aminal derivatives as potential precursors for the synthesis of amino acid under Dean-Stark water trapping conditions. Zhu et al. [24] have reported the synthesis of fluorine-containing N,N'-alkylidenebisamides in the presence of fluoroalkanesulfonic acids. Bhatnagar et al. [25, 26] have reported the synthesis of benzylidene bisamides from the direct condensation of benzaldehyde and different amide derivatives. However,most of these existing methods involve toxic metal ions and solvents,carry high costs,use corrosive reagents and require cumbersomework-up procedures. Syntheticmethodologies based on green chemistry processes are of increasing interest in organic syntheses. Recently,silica supported zinc chloride (Silzic) has been used as a solid acid catalyst in many organic transformations [27, 28, 29] and this is consistentwithour interest in using siliconbased reagents in organic syntheses [30]. Herein we wish to report the use of Silzic as a reusable solid acid catalyst for the synthesis of bis(indolyl)methanes and N,N 0-alkylidene bisamides.
2. Experimental 2.1. Typical procedure for the synthesis of bisindolylmenthanesTo a stirred mixture of aldehyde,or ketone (5 mmol),and indole (10 mmol),Silzic (0.2 g,20 mol%) was added and the mixture was allowed to stir at 100 ℃ for the total recorded time. After the completion (the reaction was monitored by TLC analysis) of the reaction,EtOAc (20 mL) was added to the reaction mixture. Then, the solid was filtered off,the filtrate was concentrated,and the residue was subjected to short column chromatography using pet.ether-EtOAc (8:2) to give pure 3a-m. The bisindolylmethane 3 are known compounds and all spectroscopic data were in agreement with literature reports [31, 32, 33].
2.2. General procedure for synthesis of N,N'-alkylidene bisamidesTo a stirred mixture of aldehyde (5 mmol),and acetamide (10 mmol),was added Silzic (0.2 g,20 mol%) and the mixture was allowed to stir at 100 ℃ for the recorded time. After the completion of the reaction (the reaction was monitored by TLC analysis),EtOH (20 mL) was added to the reaction mixture. Then,the solid was filtered off,the filtrate was concentrated,and the solid residue was washed with diethyl ether to give the pure products (4a-j). Some of the N,N 0-alkylidene bisamides 4 are known compounds and all spectroscopic data were in agreement with literature reports.
Data for a representative example are showed:
N,N ' -(4-Methoxyphenyl)methylene)diacetamide (4b): Mp 230 ℃; IR (KBr,cm-1 ): n 3276,3030,2933,2838,1671,1567, 1513,1367,1249,1183,1090,820,596; 1H NMR (300 MHz,DMSOd6): d 8.03 (d,2H,J = 7.3 Hz,2NH),7.33 (d,1H,J = 8.1 Hz,Ar-H), 6.80 (d,2H,J = 8.1 Hz,Ar-H),6.54 (t,1H,J = 7.5 Hz,CH),3.81 (s,3H, OCH3),1.79 (s,6H,2CH3); 13C NMR (75 MHz,DMSO-d6): δ 170.25, 158.5,141.3,127.2,114.2,68,=,23.2; MS: m/z (%) 236.12 (M+, 100.0),237.12 (M+ + 1,13.3); Anal. calcd. C,61.00; H,6.83; N, 11.86,found: C,60.86; H,6.43; N,11.56.
N,N'-(4-Methylphenyl)methylene)diacetamide (4d): Mp 236 ℃; IR (KBr,cm-1 ): n 3275,3031,2951,2854,1670, 1566,1541,1394,1280,1092,859,809,630; 1H NMR (300 MHz, DMSO-d6): δ 8.28 (d,2H,J = 7.6 Hz,2NH),7.14 (d,2H,J = 8.2 Hz,Ar- H),7.11 (d,2H,J = 8.6 Hz,Ar-H),6.53 (t,1H,J = 7.7 Hz,CH),2.43 (s, 3H,CH3) 1.84 (s,6H,2CH3); 13CNMR (75 MHz,DMSO-d6): δ 170.25, 140.3,136.5,128.2,124.2,68,23.2,21.2; MS: m/z (%) = 220.12 (M+, 100.0),221.12 (M+ + 1,13.7); Anal. calcd. C,65.43; H,7.32; N, 12.72,found: C,65.43; H,7.22; N,12.52.
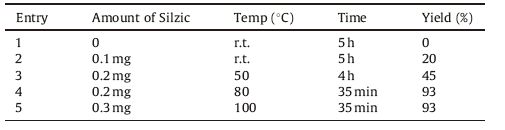
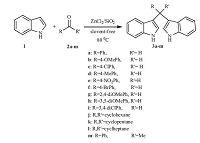
3. Results and discussionThe synthesis of bis (indolyl)methanes (3a-m) in high yields, was achieved through a reaction of indole (1) and aldehydes or ketones (2) using Silzic as depicted in Scheme 1. As a part of an ongoing study to investigate the optimum conditions for these reactions,we studied the effect of the catalyst loading at different temperatures using indole (10 mmol) and benzaldehyde (5 mmol) as a model reaction. The obtained results are summarized in Table 1.
|
|
Table 1 Optimization of the reaction conditions. |
|
Download:
|
| Scheme 1.Synthesis of bis(indolyl)methane. | |
It is found that the use of 0.2 g,20 mol% of Silzic at 80 ℃ resulted in the highest yield,and the increase of the catalyst or temperature does not lead to increased output. To investigate the scope and the generality of this new protocol, the reaction was extended to a variety of aldehydes as well as ketones with indole and the results are summarized in Table 2. Though the reactions of indole with various aldehydes were fast,the reaction with ketones took longer time (Table 2, entries 10-13). The electron deficiency and nature of the substituent on the aromatic ring affect the conversion rate,As expected the aldehydes having electron-withdrawing groups on the aromatic ring (Table 2,entries 3,5 and 6) react faster than aldehydes having electron-donating groups (Table 2,entries 2,4,7 and 8).
|
|
Table 2 Synthesis of bis(indolyl)methanes using Silzic. |
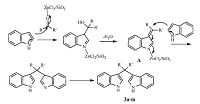
The structural elucidation of bis(indolyl)methane derivatives was assigned on the basis of melting point and spectral analyses. First,in the IR spectra of these compounds,the absorption at 3400- 3460 cm-1 attributed for the NH group,and 1335 for C-N. The 1H NMR spectrum of 3 showed a singlet at 5.83-5.91 ppm for the proton of C-H,another singlet for two N-H protons appeared at 7.85-7.94 ppm,and the aromatic protons appeared at 6.36- 7.45 ppm. For example,the 1H NMR of 3d displayed a singlet at 5.87 ppm for C-H proton,and a singlet at 7.89 ppm for two N-H protons,which disappeared with D2O exchange,in addition to the signal of a methyl group at 2.34 ppm. In addition,1H NMR of 3j showed one doublet at 2.58 and one multiplet at 1.69 ppm. These were assigned to the cyclohexane protons. The mechanism of to the Silzic-catalyzed synthesis of bisindolylmenthane is proposed as shown in Scheme 2. First,an aldehyde or ketone was activated by the Silzic and underwent an electrophilic substitution reaction at the 3-position of the indole. After dehydration,intermediate (A) was formed and was further activated by Silzic to become an electrophile,which was attacked by a second molecule of indole,to form bisindolylmenthane (3a-m).
|
Download:
|
| Scheme 2.A plausible mechanism for the formation of bis(indolyl)methane. | |
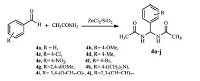
The present study has established a new,mild and convenient protocol for the synthesis of symmetrical bisamides by condensing aryl aldehydes and acetamide using Silzic as catalyst (Scheme 3, Table 3).
|
Download:
|
| Scheme 3.Synthesis of N,N'-alkylidene bisamides. | |
|
|
Table 3 Synthesis of N,N 0 -alkylidene bisamides using Silzic under solvent-free conditions. |
The experimental results illustrate the efficiency of the present method. The reactions preceded well with various aldehydes and acetamide to provide symmetrical N,N'-alkylidene bisamides. Aromatic aldehydes containing electron-withdrawing groups (such as nitro-) required shorter time and higher yield than those with electron-donating groups (such as a methoxy group). In addition,the present procedure worked well with sterically hindered aldehydes,e.g.,2,4-dimethoxy benzaldehyde,benzo[ d][1, 3]dioxole-5-carbaldehyde,and 1-naphthaldehyde and gave good yields (Table 3,entries 7,9 and 10).
The structures of the products were assigned based on the analysis of spectroscopic data such as IR,and 1 H NMR,which were found to be in agreement with the literature values [22, 34, 35, 36]. The IR spectra showed characteristic absorption bands at 3319- 3265 and 1671-1654 cm-1 for the NH group,and the carbonyl group,respectively. The 1H NMR showed a triplet at 6.52- 6.59 ppm with a coupling constant of 7.8 Hz for the CH proton (which converted to a singlet with D2O exchange),a multiplet at 6.78-7.65 ppm for the aromatic protons,and a doublet at 8.19- 8.72 ppm with J = 7.8 Hz was observed for the NH proton (which disappeared with D2O exchange). The NH proton was coupled with the CH proton with a coupling constant of 7.8 Hz.
To explain the formation of bisamides via the one-pot multicomponent reaction,we have proposed a plausible reaction mechanism,which is illustrated in Scheme 4. First,the activation of aldehyde by π empty orbital of Lewis acid occurred to form a cationic intermediate B). The second molecule of amide is added to B to produce the product. To check the reusability of catalyst,the catalyst was filtered off and washed with chloroform repeatedly,dried and reused. It was found that catalyst can be recycled at least three cycles without any change in activity.
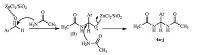
|
Download:
|
| Scheme 4.A plausible mechanism for the formation of bisamide. | |
In conclusion,Silzic has been successfully used as an effective catalyst for the synthesis of symmetrical N,N'-alkylidene bisamides for the first time. This procedure has advantages in comparison with the previously reported methods,in terms of yield,green catalyst,mild reaction conditions,simple procedures,lack of toxicity,low cost,and the use of a commercially available catalyst and simplicity of workup.
AcknowledgmentFinancial support by National Research Center (Cairo,Egypt) is gratefully appreciated. Appendix A. Supplementary data Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.11013.
| [1] | (a) A.K. Maiti, P. Bhattacharyya, Montmorillonite clay-catalysed synthesis of bis(indol-3-yl)-methanes and 1,2-bis(indol-3-yl)ethanes, J. Chem. Res. (1997) 424-425;(b) M. Chakrabarty,N. Ghosh, R. Basak, Y. Harigaya, A facile and efficient synthesis of 2,2-bis(3'/2'-indolyl)ethylamines and three bisindolic natural products, Synth. Commun. 34(2004) 421-434. |
| [2] | (a) J.B. Meng, D.M. Du, G.X. Xiong, et al., A dual pathway in the solid-state photoreaction of nitrobenzaldehydes with indole, J. Heterocyclic Chem. 31(1994) 121-124;(b) X. Li, Y. Wang, D. Du, et al., Solid state reactions of indole with carbonyl compounds, Sci. China Ser. B 40(1997) 270-277. |
| [3] | G.V.M. Sharma, J.J. Reddy, P.S. Lakshmi, P.R. Krishna, A versatile and practical synthesis of bis(indolyl)methanes/bis(indolyl)glycoconjugates catalyzed by trichloro-1,3,5-triazine, Tetrahedron Lett. 45(2004) 7729-7732. |
| [4] | H. Firouzabadi, N. (Ⅰ)ranpoor, A.A. Jafari, Aluminumdodecatungstophosphate (AlPW12O40), a versatile and a highly water tolerant green Lewis acid catalyzes efficient preparation of indole derivatives, J. Mol. Catal., A:Chem. 244(2005) 168-172. |
| [5] | M.L. Deb, P.J. Bhuyan, An efficient and clean synthesis of bis(indolyl)methanes in a protic solvent at room temperature, Tetrahedron Lett. 47(2006) 1441-1443. |
| [6] | Z.H. Zhang, L. Yin, Y.M. Wang, An efficient and practical process for the synthesis of bis(indolyl)methanes catalyzed by zirconium tetrachloride, Synthesis (2005) 1949-1954. |
| [7] | S.J. Ji, S.Y. Wang, Y. Zhang, T.P. Loh, Facile synthesis of bis(indolyl)methanes using catalytic amount of iodine at room temperature under solvent-free conditions, Tetrahedron 60(2004) 2051-2055. |
| [8] | S.J. Ji, M.F. Zhou, D.G. Gu, et al., Efficient synthesis of bis(indolyl)methanes catalyzed by lewis acids in ionic liquids, Synlett (2003) 2077-2079. |
| [9] | L.P. Mo, Z.C. Ma, Z.H. Zhang, CuBr2-catalyzed synthesis of bis(indolyl)methanes, Synth. Commun. 35(2005) 1997-2204. |
| [10] | M. Xia, S.H. Wang, W.B. Yuan, Lewis acid catalyzed electrophilic substitution of indole with aldehydes and schiff's bases under microwave solvent-free irradiation, Synth. Commun. 34(2004) 3175-3182. |
| [11] | C. Ramesh, J. Banerjee, R. Pal, B. Das, Silica supported sodium hydrogen sulfate and amberlyst-15:two efficient heterogeneous catalysts for facile synthesis of bisand tris (1H-indol-3-yl) methanes from indoles and carbonyl compounds[1], Adv. Synth. Catal. 345(2003) 557-559. |
| [12] | D.M. Pore, U.V. Desai, T.S. Thopate, P.P. Wadgaonkar, A mild, expedient, solventless synthesis of bis(indolyl)alkanes using silica sulfuric acid as a reusable catalyst, Arkivoc 12(2006) 75-80. |
| [13] | K. Niknam, M.A. Zolfigol, T. Sadabadi, A. Nejati, Preparation of indolylmethanes catalyzed by metal hydrogen sulfates, J. (Ⅰ)ran. Chem. Soc. 3(2006) 318-322. |
| [14] | L.P. Zhang, Y.Q. Li, M.Y. Zhou, Efficient and eco-friendly process for the synthesis of bi (indolyl)-methanes catalyzed by sodium hydrogensulfate monohydrate in ionic liquid n-butylpyridinium tetrafluoroborate, Chin. Chem. Lett. 17(2006) 723-726. |
| [15] | C. Ramesh, N. Ravindranath, B. Das, Electrophilic substitution reactions of indoles with carbonyl compounds using ceric ammonium nitrate:A novel and efficient method for the synthesis of di-and tri-indolylmethanes, J Chem. Res. Synop. (2003) 72-74. |
| [16] | H. Koshima, W. Matsuaka, N-bromosuccinimide catalyzed condensations of indoles with carbonyl compounds under solvent-free conditions, J. Heterocycl. Chem. (2002) 1089-1091. |
| [17] | A. Khalafi-Nezhad, A. Parhami, A. Zare, et al., Trityl chloride as a novel and efficient organic catalyst for room temperature preparation of bis(indolyl)methanes under solvent-free conditions in neutral media, Synthesis (2008) 617-621. |
| [18] | J.W. Bode, Emerging methods in amide-and peptide-bond formation, Curr. Opin. Drug Discovery Dev. 9(2006) 765-775. |
| [19] | H.R. Shaterian, M. Ghashang, M. Feyzi, Silica sulfuric acid as an efficient catalyst for the preparation of 2H-indazolo[2,1-b]phthalazine-triones, Appl. Catal., A:Gen. 345(2008) 128-133. |
| [20] | T. Yamazaki, K.(Ⅰ). Nunami, M. Goodman, Cyclic retro-inverso dipeptides with two aromatic side chains. Ⅱ. Conformational analysis, Biopolymers 31(1991) 1513-1528. |
| [21] | M. Goodman, H. Shao, Peptidomimetic building blocks for drug discovery:an overview, Pure Appl. Chem. 68(1996) 1303-1308. |
| [22] | N.P. Selvam, S. Saranye, P.T. Perumal, A convenient and efficient protocol for the synthesis of symmetrical N,N'-alkylidine bisamides by sulfamic acid under solvent-free conditions, Can. J. Chem. 85(2008) 32-38. |
| [23] | A. Milenkovic, F. Fache, R. Faure, M. Lemaire, Activated imines and aminal derivatives:potential precursors of beta-amino acids, Synth. Commun. 29(1999) 1535-1546. |
| [24] | S. Zhu, G. Xu, Q. Chu, Y. Xu, C. Qui, Synthesis of fluorine-containing symmetrical N,N-alkylidene bisamides, J. Fluorine Chem. 93(1999) 69-71. |
| [25] | G.S. Bhatnagar, K.C. Pandya, Condensation of aldehydes with amides, Proc.-(Ⅰ)ndian Acad. Sci., Sect. A 24(1946) 487-495. |
| [26] | R.K. Mehra, K.C. Pandya, The condensation of aldehydes with amides, Proc.-(Ⅰ)ndian Acad. Sci., Sect. A 10(1939) 285-288. |
| [27] | D.J. Upadhya, S.D. Samant, A facile and efficient pinacol-pinacolone rearrangement of vicinal diols using ZnCl2 supported on silica as a recyclable catalyst, Appl. Catal., A:Gen. 340(2008) 42-51. |
| [28] | H.A. Soliman, A.Y. Mubarak, A. El-Mekabati, S.S. Elmorsy, SiO2/ZnCl2-catalyzed heterocyclic synthesis:green, rapid and efficient one-pot synthesis of 14-Hdibenzo[a,j]xanthenes, 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines under solvent-free conditions, Chem. Sci. Trans. 3(2014) 819-825. |
| [29] | H.A. Soliman, A.Y. Mubarak, A. El-Mekabati, H.M. Awad, S.S. Elmorsy, Ecofriendly synthesis of amidochloroalkyl naphthols, cyclization of the product to oxazepinones for biological evaluation, Monatsh Chem. (2015), http://dx.doi.org/10.1007/s00706-015-1536-2. |
| [30] | (a) H.A. Soliman, F.M.E. Abdel-Megeid, S.S. Elmorsy, Silicon tetrachloride-induced regioselective reaction of N-bromosuccinimide with arylidene malononitrile and with α,β-unsat-urated ketones, Org. Chem.:An (Ⅰ)ndian J. 11(2015) 223-228;(b) H.A. Soliman, T.K. Khatab, Efficient heterogeneous catalytic one-pot, three component synthesis of γ-hydroxy-β-ketoamide, Egypt. J. Chem. 57(2014) 129-142;(c) H.A. Soliman, T.A. Salama, Silicon tetrachloride-induced green and efficient MCR protocol for the synthesis of 1,8-dioxo-decahydroacridines and their transformation to novel functionalized pyrido-tetrazolo[1,5-a]azepine derivatives, Org. Chem.:An (Ⅰ)ndian J. 10(2014) 63-68;(d) T.K. Khatab, K.A.M. El-Bayouki, W.M. Basyouni, A new and facile tetrachlorosilane-promoted one-pot condensation for the synthesis of a novel series of tetracyclic 1,5-thiazepines, Tetrahedron Lett. 55(2014) 6039-6041;(e) H.A. Soliman, T.A. Salama, Silicon-mediated highly efficient synthesis of 1,8-dioxo-octahydroxanthenes and their transformation to novel functionalized pyrano-tetrazolo[1,5-a] azepine derivatives, Chin. Chem. Lett. 24(2013) 404-406;(f) T.K. Khatab, K.A.M. El-Bayouki, W.M. Basyouni, An efficient synthesis of bacylureas via a three-component, one-pot synthesis using TCS/ZnCl2, Tetrahedron Lett. 52(2011) 1448-1451. |
| [31] | V.T. Kamble, B.P. Bandgar, S.N. Bavikar, Highly efficient synthesis of bis(indolyl)-methanes catalyzed by sodium tetrafluoroborate, Chin. J. Chem. 25(2007) 13-17. |
| [32] | S. Sheng, Q. Wang, Y. Ding, X. Liu, M. Cai, Synthesis of bis(indolyl)methanes using recyclable PEG-supported sulfonic acid as catalyst, Catal. Lett. 128(2009) 418-422. |
| [33] | R. Ghorbani-Vaghei, H. Veisi, H. Keypour, A.A. Dehghani-Firouzabadi, A practical and efficient synthesis of bis(indolyl)methanes in water, and synthesis of di-, tri-, and tetra(bis-indolyl)methanes under thermal conditions catalyzed by oxalic acid dehydrate, Mol. Divers 14(2010) 87-96. |
| [34] | M.R.M. Shafiee, Silica-supported barium chloride (SiO2-BaCl2)-efficient and heterogeneous catalyst for the environmentally friendly preparation of N,N'-alkylidene bisamides under solvent-free conditions, Can. J. Chem. 89(2011) 555-561. |
| [35] | M.R.M. Shafiee, One-pot preparation of N,N'-alkylidene bisamide derivatives catalyzed by silica supported polyphosphoric acid (SiO2-PPA), J. Saudi Chem. Soc. 18(2014) 115-119. |
| [36] | B.F. Mirjalili, M.A. Mirhoseini, Tandem synthesis of N,N-alkylidenebisamides promoted by nano-SnCl4.SiO2, J. Chem. Sci. 125(2013) 1481-1486. |
 2016, Vol.27
2016, Vol.27