Quinolines [1, 2],a versatile class of heterocyclic compounds,are found extensively in natural products [3] and frequently investigated for their broad biological properties [4, 5, 6, 7, 8, 9, 10, 11, 12, 13],such as antitumor activities [10, 11] and HIV inhibitory potencies [12, 13]. Thus,many synthetic approaches have been reported and a large number of quinolines are synthesized [14, 15, 16, 17, 18]. However,the synthesis of acenaphtho[1,2-b]quinolines has rarely been reported in the literature. Therefore,the development of a novel and efficient approach to construct this fused heterocyclic scaffold is highly desirable.
Compared to other strategies,a cascade strategy [19, 20, 21, 22] could conveniently and quickly assemble the chemical skeletons of quinolines with metal catalysts [23, 24] playing important roles in the chemical reactions. The synthesis of dihydrobenzo-[4, 5][1, 3]oxazino[2,3-a]isoquinolines from 2-aminobenzyl alcohol and 2-phenylethynyl-benzaldehyde was reported recently [25, 26, 27, 28],in which the metal-catalyzed cascade reaction performed fundamental roles. As a continuation of our previous work [29],we hypothesize that the coupling reactions of 8-substituted phenylethynyl-1-naphthaldehydes 1 with substituted anilines 2 through an intermolecular condensation could provide the corresponding imines,which could subsequently proceed to acenaphtho[1,2-b]quinoline derivatives in the presence of appropriate catalysts in an apparently simple process (Scheme 1). Herein,we report the successful realization of this concept by utilizing CuI as a catalyst. The cascade transformation proceeds with broad array of substrates providing diverse analogs of 8-substituted phenylethynyl-1-naphthaldehydes 1 and substituted anilines 2.
|
Download:
|
| Scheme 1.Concept of metal-catalyzed cascade reactions. | |
General procedure for synthesis of 3a-m: To a stirred solution of 8-substituted phenylethynyl-1-naphthaldehydes 1 (0.1 mmol) and CuI (0.02 mmol) in 1,2-dichloroethane (DCE,10 mL),substituted anilines 2 were added. The resulting mixture was heated to reflux for 12 h. After the reaction was completed,the mixture was concentrated under reduced pressure,and the obtained residue was purified by column chromatography (PE/EA = 10:1-3:1) to give the corresponding compounds 3a-m in yields of 46%-76%. All compounds were confirmed by 1 H NMR,13C NMR and HRMS.
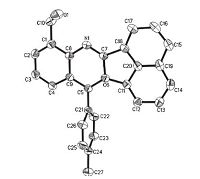
Additionally,3b was further characterized by X-ray crystallography (Fig. 1). Single crystal data (CCDC 1416928) has been deposited in the Cambridge Crystallographic Data Centre. Details of spectral data are provided in Supporting information.
|
Download:
|
| Fig. 1.ORTEP drawing of X-ray structure of 3b. | |
(12-Phenyl-acenaphtho[1,2-b]quinolin-8-yl)-methanol 3a: Yield (27 mg,75%),mp 183-184 ℃. 1H NMR (500 MHz,CDCl3): d 8.46 (d,1H,J = 6.5 Hz),8.04 (d,1H,J = 8.0 Hz),7.88 (d,1H,J = 8.0 Hz),7.83 (t,1H,J = 7.2 Hz),7.68-7.71 (m,3H),7.60-7.64 (m,2H),7.57-7.= (m,2H),7.41-7.46 (m,2H),6.92 (d,1H,J = 7.0 Hz),5.61 (brs,1H),5.39 (s,2H). 13C NMR (125 MHz,CDCl3): δ 158.4,142.9,138.3,136.2,135.5,134.7,133.6,130.1,129.3,129.2,129.1,129.05,128.7,128.4,128.2,127.7,127.6,127.3,
126.98,126.3,125.9,122.7,121.4,65.46. HRMS (TOF-ESI): [M + H]+,calcd. for C26H18NO: 360.1383. found: 360.1379.
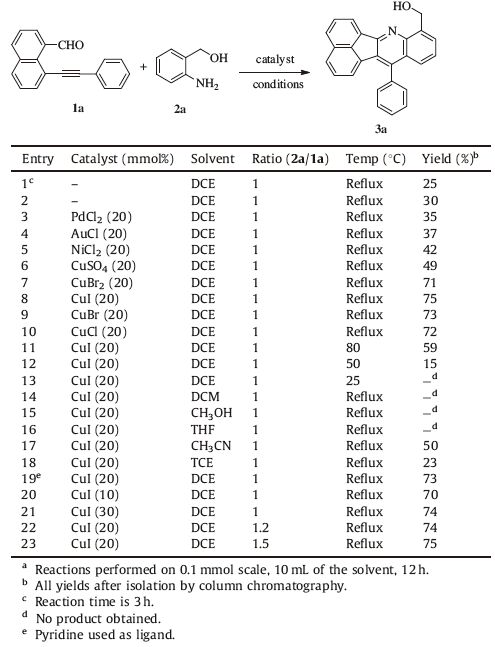
Initially,our efforts focused on discovering the optimal reaction conditions to perform the proposed reaction. All results are summarized in Table 1. Preliminary experiments to optimize the reaction conditions were performed with 8-phenylethynyl-1-naphthaldehyde 1a and 2-aminobenzyl alcohol 2a as model substrates. When the reaction was initially performed using DCE as the solvent at the reflux temperature for 3 h in the absence of the catalyst,a new fluorescent spot emerged upon TLC analysis. The product was subsequently isolated and confirmed to be the desirable cascade product 3a by 1 H NMR and 13C NMR in a low yield (25%,Table 1,entry 1). Furthermore,the yield improved slightly to 30% with the increase of the reaction time from 3 h to 12 h (Table 1,entry 2). According to the report of Patil et al. [25],AgNO3 and PdCl2 were efficient catalysts for cascade reactions involving the synthesis of dihydrobenzo [4, 5][1, 3]oxazino[2,3-a]isoquinolines,and thus we searched for the most suitable metallic catalyst. Copper ion [30, 31] was identified as effective in the screening of metallic catalysts (Table 1,entries 3-10). Among them,CuI [32, 33] was proved to be one of the most optimal catalysts for this cascade reaction,increasing the yield up to 75% (Table 1,entry 8). Apparently,the solvent exerted a significant influence on the yield of the reactions (Table 1,entries 14-18). DCE was found to be most suitable in the preliminary screening.
|
|
Table 1 Optimization of the reaction conditionsa |
Pyridine,a nitrogen-containing ligand,was investigated to attempt to further improve the efficiency,but it failed (Table 1,entry 19). Subsequently,we adjusted the reaction temperature to 80 ℃ and 50 ℃ However,these changes decreased the yield to 59% and only 15%,respectively (Table 1,entries 11,12) and no product was observed when the reaction temperature was further lowered to room temperature (Table 1,entry 13). Subsequent variations in the amount of CuI (Table 1,entries 20,21) and 1a/2a molar ratio (Table 1,entries 22,23),did not further improve the reaction efficiency.
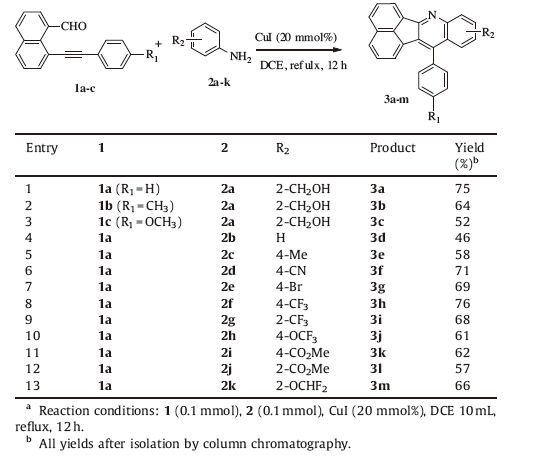
Having established the most favorable reaction conditions,we then attempted to investigate the extend and limitations of this cascade strategy by exploring a variety of starting materials with structural variations of both 2/4-substituted anilines 2a-k and 8-substituted phenylethynyl-1-naphthadehyde 1a-c. Notably as shown in Table 2,all tested substrates were successful tolerated in the cascade approach and the corresponding analogs of acenaphtho[1,2-b]quinolines 3a-m were produced in moderate to good yield (46%-76%). The cascade reaction of 1b and 2a provided 3b in 64% yield (Table 2,entry 2). However,introduction of a methoxy group on the phenyl ring in the 8-substituted phenylethynyl-1-naphthaldehyde 1c decreased the yield (52%),presumably due to the influence of electronic effects (Table 2,entry 3). The reaction of 1 with para-substituted anilines 2d-f,2h-2i generated the corresponding products in good yields (Table 2,entries 6-8,10 and 11). The reaction of 1a with ortho-substituted anilines 2a,2g and 2j-k also gave good yields of the corresponding products (entries 1,9,12 and 13). In addition,alkylethynyl-1-naphthadehydes (changing phenyl to alkyl in 1a) were also investigated in this protocol,however,the results were poor.
|
|
Table 2 Scopes and limitations of the cascade reactiona. |
A plausible mechanism for this cascade transformation is proposed in Scheme 2,similar to that reported in the study of Patil et al. [25]. The condensation of 1b and 2a generates the key imine intermediate 4,which subsequently converts into the final product 3b via two possible catalytic pathways (A and B). Under pathway A,the imine intermediate 4 is activated by CuI to form an iminoalkyne Cu-complex 5,which further generates the target scaffold 3b through an intramolecular nucleophilic addition. In the conceivable alternative pathway B,the π-Cu complex 6 is probably formed due to the activation of the intermediate 4 by CuI,which is subject to a subsequent intramolecular nucleophilic addition to afford the target compound 3b.
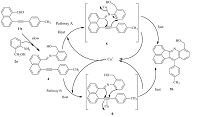
|
Download:
|
| Scheme 2.A plausible mechanism for the CuI-catalyzed cascade reaction. | |
In conclusion,we have now established an efficient CuIcatalyzed cascade reaction for the one-pot synthesis of acenaphtho[1,2-b]quinoline analogs. The broad functional group compatibility of this reaction could be a powerful tool for the convenient and quick synthesis of novel quinoline derivatives in drug discovery.
AcknowledgmentWe gratefully acknowledge the National Key Technology R&D Program"New Drug Innovation" of China (No. 2013ZX09402103)
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2015.11.011.
| [1] | K.W. Bentley, β-Phenylethylamines and the isoquinoline alkaloids, J. Nat. Prod. Rep. 22(2005) 249-268. |
| [2] | M. Chrzanowska, M.D. Rozwadowska, Asymmetric synthesis of isoquinoline alkaloids, Chem. Rev. 104(2004) 3341-3370. |
| [3] | K.W. Bentley, β-Phenylethylamines and the isoquinoline alkaloids, J. Nat. Prod. Rep. 23(2006) 444-463. |
| [4] | R.S. Keri, S.A. Patil, Quinoline:a promising antitubercular target, Biomed. Pharmacother. 68(2014) 1161-1175. |
| [5] | Y. Sheng, B. Sun, X. Xie, et al., DMH1(4-[6-(4-isopropoxyphenyl)pyrazolo[1,5-a]pyrimidin-3-yl]quinoline) inhibits chemo-therapeutic drug-induced autophagy, Acta Pharm. Sin. B 5(2015) 330-336. |
| [6] | B. Gryzlo, K. Kulig, Quinoline-a promising fragment in the search for new antimalarials, Mini-rev. Med. Chem. 14(2014) 332-344. |
| [7] | P. Zajdel, A. Partyka, K. Marciniec, et al., Quinoline-and isoquinoline-sulfonamide analogs of aripiprazole:novel antipsychotic agents? Future Med. Chem. 6(2014) 57-75. |
| [8] | S. Mukherjee, M. Pal, Quinolines:a new hope against inflammation, Drug Discov. Today 18(2013) 389-398. |
| [9] | K. Kaur, K.M. Jain, R.P. Reddy, R. Jain, Quinolines and structurally related heterocycles as antimalarials, Euro. J. Med. Chem. 45(2010) 3245-3264. |
| [10] | A.D.C. Parenty, L.V. Smith, K.M. Guthrie, et al., Highly stable phenanthridinium frameworks as a new class of tunable DNA binding agents with cytotoxic properties, J. Med. Chem. 48(2005) 4504-4506. |
| [11] | V.R. Solomon, H. Lee, Quinoline as a privileged scaffold in cancer drug discovery, Curr. Med. Chem. 18(2011) 1488-1508. |
| [12] | K. (Ⅰ)wasa, M. Moriyasu, Y. Tachibana, et al., Simple isoquinoline and benzylisoquinoline alkaloids as potential antimicrobial, antimalarial, cytotoxic, and anti-H(Ⅰ)V agents, Bioorg. Med. Chem. 9(2001) 2871-2884. |
| [13] | M.V. Reddy, M.R. Rao, D. Rhodes, et al., Lamellarin α 20-sulfate, an inhibitor of H(Ⅰ)V-1 integrase active against H(Ⅰ)V-1 virus in cell culture, J. Med. Chem. 42(2009) 1901-1907. |
| [14] | R. Khusnutdinov, A. Bayguzina, R. Aminov, U. Dzhemilev, Quinoline synthesis by the reaction of anilines with 1,2-diols catalyzed by iron compounds, J. Heterocycl. Chem. (2015), http://dx.doi.org/10.1002/jhet.2425. |
| [15] | X. Mi, J. Chen, L. Xu, FeCl3-catalyzed SF5-containing quinoline synthesis:threecomponent coupling reactions of SF5-anilines, aldehydes and alkynes, Eur. J. Org. Chem. 2015(2015) 1415-1418. |
| [16] | C.H. Tseng, C.K. Lin, Y.L. Chen, et al., Synthesis, antiproliferative and anti-dengue virus evaluations of 2-aroyl-3-arylquinoline derivatives, Euro. J. Med. Chem. 79(2014) 66-76. |
| [17] | M.H. Son, J.Y. Kim, E.J. Lim, et al., Synthesis and biological evaluation of 2-(arylethynyl)quinoline derivatives as mGluR5 antagonists for the treatment of neuropathic pain, Bioorg. Med. Chem. Lett. 23(2013) 1472-1476. |
| [18] | P. Szymanski, A. Laznickova, M. Laznicek, et al., 2,3-Dihydro-1H-cyclopenta[b]-quinoline derivatives as acetylcholinesterase inhibitors-synthesis, radiolabeling and biodistribution, (Ⅰ)nt. J. Mol. Sci. 13(2012) 10067-10090. |
| [19] | S.L. Schreiber, Target-oriented and diversity-oriented organic synthesis in drug discovery, Science 287(2000) 1964-1969. |
| [20] | C.J. O'Connor, H.S.G. Beckmann, D.R. Spring, Diversity-oriented synthesis:producing chemical tools for dissecting biology, Chem. Soc. Rev. 41(2012) 4444-4456. |
| [21] | L.Q. Lu, J.R. Chen, W.J. Xiao, Development of cascade reactions for the concise construction of diverse heterocyclic architectures, Acc. Chem. Res. 45(2012) 1278-1293. |
| [22] | J.M. Smith, J. Moreno, B.W. Boal, N.K. Garg, Cascade reactions:a driving force in akuammiline alkaloid total synthesis, Angew. Chem. (Ⅰ)nt. Ed. 54(2015) 400-412. |
| [23] | J.H. Xie, D.H. Bao, Q.L. Zhou, Recent advances in the development of chiral metal catalysts for the asymmetric hydrogenation of ketones, Synthesis 47(2015) 460-471. |
| [24] | E.G. Chepaikin, Oxidative functionalization of alkanes under dioxygen in the presence of homogeneous noble metal catalysts, J. Mol. Catal. A-Chem. 85(2014) 160-174. |
| [25] | N.T. Patil, A.K. Mutyala, G.V.V. Lakshmi, P.V.K. Raju, B. Sridhar, Facile assembly of fused isoquinolines by gold ((Ⅰ))-catalyzed coupling-cyclization reactions between o-alkynylbenzaldehydes and aromatic amines containing tethered nucleophiles, Eur. J. Org. Chem. 2010(2010) 1999-2007. |
| [26] | B. Jiang, Y. Zhou, Q. Kong, et al., ‘One-pot’ synthesis of dihydrobenzo[4,5][1,3] oxazino[2,3-a]isoquinolines via a silver ((Ⅰ))-catalyzed cascade approach, Molecules 18(2013) 814-831. |
| [27] | N.T. Patil, A. Konala, S. Sravanti, et al., Electrophile induced branching cascade:a powerful approach to access various molecular scaffolds and their exploration as novel anti-mycobacterial agents, Chem. Commun. 49(2013) 10109-10111. |
| [28] | A.K. Verma, D. Choudhary, R.K. Saunthwal, et al., On water:silver-catalyzed domino approach for the synthesis of benzoxazine/oxazine-fused isoquinolines and naphthyridines from o-alkynyl aldehydes, J. Org. Chem. 78(2013) 6657-6669. |
| [29] | Y.F. Tong, S.P. Wang, Q.Y. Yang, Synthesis of 8-iodo-1-naphthaldehyde, Chem. Reagent. 35(2013) 455-456(p.460). |
| [30] | L.K. Kong, Y.Y. Zhou, H. Huang, et al., Copper-catalyzed synthesis of substituted quinolines via C-N coupling/condensation from ortho-acylanilines and alkenyl iodides, J. Org. Chem. 80(2015) 1275-1278. |
| [31] | B. Li, C.H. Guo, X.S. Fan, J. Zhang, X.Y. Zhang, Synthesis of substituted quinoline via copper-catalyzed one-pot cascade reactions of 2-bromobenzaldehydes with aryl methyl ketones and aqueous ammonia, Tetrahedron Lett. 55(2014) 5944-5948. |
| [32] | S.B. Paul, K.C. Majumdar, S. Anwar, S. Choudhury, Copper((Ⅰ)) iodide supported synthesis of coumarin and quinolone annulated 2-aminothiazoles, Synlett 26(2015) 1039-1044. |
| [33] | H.C. Ouyang, R.Y. Tang, P. Zhong, Cu(Ⅰ)/(Ⅰ)2-promoted electrophilic tandem cyclization of 2-ethynylbenzaldehydes with ortho-benzenediamines:synthesis of iodoisoquinoline-fused benzimidazoles, J. Org. Chem. 76(2011) 223-228. |
 2016, Vol.27
2016, Vol.27