b. Oil & Gas Field Applied Chemistry Key Laboratory of Sichuan Province, School of Chemistry and Chemical Engineering, Southwest Petroleum University, Chengdu 610500, China
In the recent years,thiazolidinones have been widely investigated and have fascinated medicinal and organic chemists. The 4- thiazolidinones are especially important structural units of heterocyclic compounds exhibiting a variety of biological effects, such as anti-inflammatory [1],antibacterial [2],antiviral [3], antitumor [4],antimalarial [5],anti-HIV [6],tuberculostatic [7], antihistamine [8],anticonvulsant [9],and antiarrythmic [10]. During the past few years,many synthetic methods have been successfully developed to accomplish the condensation and cyclization reaction. However,the majority of catalysts used in these reactions are metal Lewis acid catalysts (ZnCl2,iron salts) [11],sodium sulfate [12],coupling reagents,like DCC [13] and HBTU [14],molecular sieves [15] and others [16] that have also been documented for the synthesis of 2,3-disubstituted 4- thiazolidinones. Among these,the processes catalyzed by molecular sieves are notable examples because they are environmentally friendly,low cost,allow efficient catalyst recovery and recyclability. To the best of our knowledge,in recent research most of the heterogeneous catalysts are mainly restricted to the discussion of synthesis and characterization,and only a small number of examples have been described in organic catalysis. To explore new types of heterogeneous catalysts in available templates for organocatalytic reactions,we recently explored the use of defined mesoporous siliceous material (MCM-41) on which MCM-41 supported catalysts are employed to promote the Michael addition [17],Knoevenagel condensation reaction [18] and others [19]. Herein,we extend the use of the MCM-41 and describe mesoporous MCM-41 supported Schiff base and CuSO4-5H2O for the cyclocondensation of mercaptoacetic acid with imines (or aldehydes and amines) in high yields (up to 98% and 99%).
2. ExperimentalThe purity of compounds was checked by thin layer chromatography (TLC) using ethyl acetate/petroleumether (1/10-1/5,v/v) as an eluent,and IR spectrum recorded on a Bruker Equinox = FTIR spectrophotometer as KBr discs. The 1H NMR and 13C NMR spectra were recorded on an INOVA (400 MHz) FT-NMR spectrometer in DCl3 using TMS as an internal standard. The prepared ligands were characterized by FT-IR XRD,TEM (Fig. 1) and SEM (Fig. 2). These TEM and SEM images indicated that the uniformity of the particle size is subsequently reduced after the grafting of an organic moiety on the inner pore walls of MCM-41.

|
Download:
|
| Fig. 1.The transmission electron microscope (TEM) images of ligands (L1,MCM-41; L2,amine functionalized MCM-41; L3,Schiff base supported MCM-41). | |

|
Download:
|
| Fig. 2.The scanning electron micrograph (SEM) images of ligands (L1,MCM-41; L2,amine functionalized MCM-41; L3,Schiff base supported MCM-41) | |
A mixture of ligand L3 (0.005 g,0.01 mmol) and CuSO4-5H2O (0.001 g,0.05 mmol) in toluene (0.5 mL) was stirred magnetically and then heated for 0.5 h at 110 ℃ reflux. Then,imine (0.018 g, 0.1 mmol) and mercaptoacetic acid (0.014 g,0.15 mmol) were added. The progress of the reaction was monitored by TLC using ethyl acetate/petroleum ether (1/10-1/5). Upon completion of the reaction,the crude mixture was purified by column chromatography on silica gel (ethyl acetate/petroleum ether) to afford the desired pure product (3a) in 97% yield.
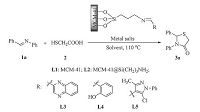
3. Results and discussionRecently,we disclosed an interesting heterogeneous catalyst generated from MCM-41,3-(triethylsilyl)propan-1-amine (NH2CH2CH2CH2Si(OEt)3),and heterocyclic aldehyde,in which the surface area and pore of MCM-41 would exhibit high reactivity during the catalyst formation (Scheme 1). As the catalytic property could be facilely tuned by altering organic functional groups on the mesoporous,this catalyst system proved generally efficient for the Knoevenagel condensation reaction and Fridel-Crafts reaction. Encouraged by these features,we desired to extend this catalyst system to the synthetic of thiazolidinones.
|
Download:
|
| Scheme 1.The cyclocondensation of imine with mercaptoacetic acid. | |
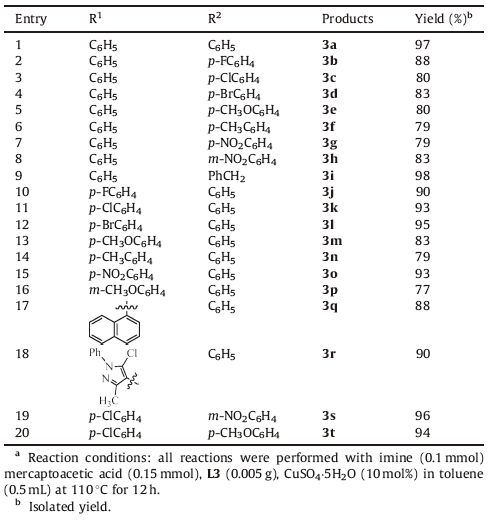
Before examining the reaction of imines and mercaptoacetic acid,it is reasonable to test the potential of the MCM-41-Schiff base ligands in the reaction. The results are summarized in Table 1. At the outset,a series of ligands catalysts,such as L1-L5,were screened in the presence of imine/mercaptoacetic acid (1/1.5)in toluene (entries 2-6),and the corresponding products were identified. Ligand L3 proved to be the best ligand for the reaction (entry 4). The sequential investigations on the metal salts,and the result obtained with CuSO4-5H2O was superior to ZnSO4-7H2O and NiSO4-7H2O in terms of yield,97%,83% and 91% yield (entries 9, 7 and 8,respectively). Then,the solvent effects on reactivity were examined in the presence of 0.005 g L3 and 10 mol% CuSO4-5H2O. And it determined that among the screened solvents,toluene was superior to other solvents. Furthermore,when the reaction was performed in polar solvents,the formation of 3a was detected in ethanol or H2O with low yields. Additional optimization of the reaction system revealed that 12 h,110 ℃ and an imine/ mercaptoacetic acid ratio of = 1/1.5 were the optimal choices. Therefore,the optimal conditions were as follows: imine (0.10 mmol),mercaptoacetic acid (0.15 mmol),L3 (0.005 g), CuSO4-5H2O (10 mol%) in toluene (0.5 mL) at 110 ℃ for 12 h.
|
|
Table 1 Optimization of the reaction conditionsa |
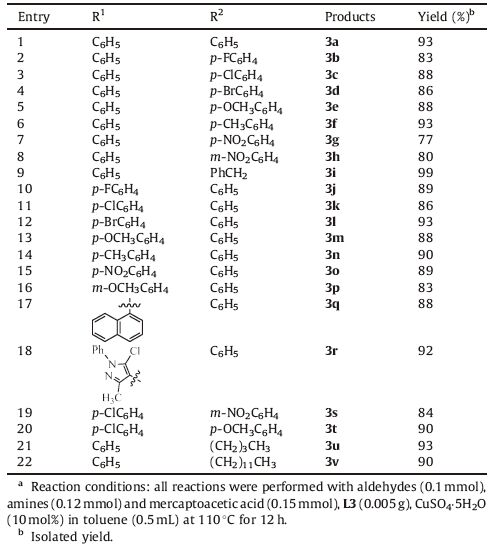
Under optimized conditions,the substrate range of imines was investigated using 0.005 g L3 and 10 mol% CuSO4-5H2O as catalyst(Scheme 2). As shown by the results in Table 2,the catalytic cyclocondensation reaction proceeded well for many differently substituted imines,independent of the electron-withdrawing or electron-donating character of the substituent (up to 97% yield). Notably,an excellent result of 98% yield was obtained when the R2 is a benzyl group,(entry 9). Moreover,the R1 substituents containing electron-attracting groups had a slightly higher reaction activity than these with electron-donating groups (entries 10-12 and 15 vs. 13-14 and 16). In addition,fused ring and heteroaromatic substituted imines also reacted well with mercaptoacetic acid to deliver the desired product in 88% and 90% yields,respectively (entries 17-18).
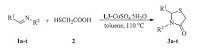
|
Download:
|
| Scheme 2.The cyclocondensation of two-component. | |
|
|
Table 2 Substrates investigated range for the cyclocondensation reaction of two-componenta |
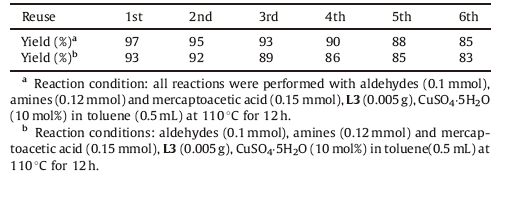
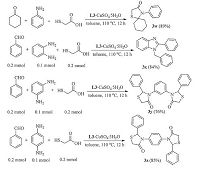
We then turned our attention to the three component reactions in the presence of 0.005 g L3 and 10 mol% CuSO4-5H2O. Various aldehydes and amines were also evaluated (Scheme 3),giving the corresponding 4-thiazolidinones with good results (up to 99%). As shown in Table 3,the reactivity was found to be insensitive to the electronic properties of the substituent of amines (entries 1-9 and 21-22),and electron-donating groups on the phenyl ring or aliphatic amines provided results with high yields (-90%). Consequently,the cyclohexanone was subjected to the reaction and yielded 3w with good result (89%). Notably,the diaminobenzene was also tested under the reaction conditions of an aldehydes:amines:mercaptoacetic acid ratios of 2:1:2. The thiazolidinone product could not be detected with the o-diaminobenzene, but 1-benzyl-2-phenyl-1H-benzo[d]imidazole 3x was obtained with 84% yield. The p- and m-diaminobenzenes could be converted into the di-thiazolidinones 3y and 3z (Scheme 4) [20].

|
Download:
|
| Scheme 3.The cyclocondensation of three-component. | |
|
Download:
|
| Scheme 4.The cyclocondesation of thioglycolic acid with ketone or diamines. | |
|
|
Table 3 Substrates investigated range for the cyclocondensation reaction of three-componenta |
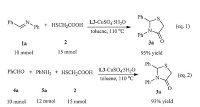
To further exploit the synthetic potential of the current catalytic system,a scaled-up synthesis of the product 3a was performed by the two-component and three-component reactions. As shown in Scheme 5,by treatment of 10 mmol of imine under the optimized reaction conditions,2.43 g of the product 3a was obtained in the two-component reaction. And in the three-component reaction, the 100-fold amount of 4a also reacted well to deliver the desired product in 93% yield without any significant loss in yield. As recyclability is important for the applications of a heterogeneous catalyst,reuse performance of mesoporous MCM-41 supported Schiff base and CuSO4-5H2O is investigated in the two-component and three-component cyclocondensation (Table 4). Upon completion of reaction,the catalyst is recovered by centrifugal separation,washed and dried and the loss of catalyst refilled and reused for next cycle. It was found that the catalyst L3/ CuSO4-5H2O exhibited good catalytic activity with 85% and 83% yields after the sixth recycle.
|
Download:
|
| Scheme 5. Scaled-up version of the cyclocondensation in the two-component and three-component reaction | |
|
|
Table 4 Reusability of L3/CuSO4-5H2O catalyzed the cyclocondensation recation. |
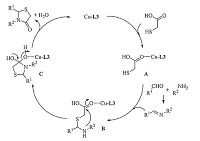
We hypothesize that mesoporous MCM-41 supported Schiff base and CuSO4-5H2O (L3-CuSO4-5H2O) may activate the acid group by forming O-Cu bonding (A) with carbonyl oxygen,and then accelerate to enhance nucleophilicity of mercapto group of thioglycolic acid causing its facile addition on the imine (B). This then facilitates the intramolecular cyclocondensation to form intermediate C. Finally,the loss of H2O affords the desired thiazolidinones (Scheme 6) [20].
|
Download:
|
| Scheme 6.Proposed catalytic mechanism. | |
In summary,the efficient synthesis of thiazolidinones derived from two-component and three-component cyclocondensation with the catalyst mesoporous MCM-41 supported Schiff base and CuSO4-5H2O is described. The results are illustrative of the ligands in the catalytic system that plays a key role in the cyclocondensation reaction. In particular,this method has the advantages of a high atomic efficiency and high yields of products (up to 98% and 99%),mild reaction conditions,simple operation,environmentally friendly and wide range of applicable substrates. Further investigations of cyclocondensation catalyzed by mesoporous MCM-41 supported Schiff base and CuSO4-5H2O are in progress.
AcknowledgmentsWe acknowledge the National Natural Science Foundation of China (Nos. 21162026,21262034 and 21362036) and Doctoral Fund of Xinjiang University (No. BS110111) for financial support, Xinjiang University Analytical & Testing Center for instrumental analyses,and Adamas-beta Chemical Co.,for all chemical reagents.
| [1] | (a) M.G. Vigorita, R. Ottana, F. Monforte, et al., Synthesis and antiinflammatory, analgesic activity of 3,3'-(1,2-ethanediyl)-bis[2-aryl-4-thiazolidinone] chiral compounds, Bioorg. Med. Chem. Lett. 11(2001) 2791-2794;(b) C. Perez, J.P. Monserrat, Y. Chen, et al., Exploring hydrogen peroxide responsive thiazolidinone-based prodrugs, Chem. Commun. 51(2015) 7116-7119. |
| [2] | (a) V.S. Palekar, A.J. Damle, S.R. Shukla, Synthesis and antibacterial activity of some novel bis-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and bis-4-thiazolidinone derivatives from terephthalic dihydrazide, Eur. J. Med. Chem. 44(2009) 5112-5116;(b) E. Pitta, E. Tsolaki, A. Geronikaki, et al., 4-Thiazolidinone derivatives as potent antimicrobial agents:microwave-assisted synthesis, biological evaluation and docking studies, Med. Chem. Commun. 6(2015) 319-326;(c) R.V. Patel, S.W. Park, Discovery of the highly potent fluoroquinolone-based benzothiazolyl-4-thiazolidinone hybrids as antibacterials, Chem. Biol. Drug Des. 84(2014) 123-129. |
| [3] | (a) J.L. Romine, D.R. St. Laurent, J.E. Leet, et al., (Ⅰ)nhibitors of HCV NS5A:from iminothiazolidinones to symmetrical stilbenes, ACS Med. Chem. Lett. 2(2011) 224-229;(b) K.M. Hosamani, R.V. Shingalapur, Synthesis of 2-mercaptobenzimidazole derivatives as potential anti-microbial and cytotoxic agents, Arch. Pharm. 344(2011) 311-319. |
| [4] | (a) D. Havrylyuk, B. Zimenkovsky, O. Vasylenko, et al., Synthesis of novel thiazolone-based compounds containing pyrazoline moiety and evaluation of their anticancer activity, Eur. J. Med. Chem. 44(2009) 1396-1404;(b) S. Wang, Y. Zhao, G. Zhang, et al., Design, synthesis and biological evaluation of novel 4-thiazolidinones containing indolin-2-one moiety as potential antitumor agent, Eur. J. Med. Chem. 46(2011) 3509-3518. |
| [5] | V.R. Solomon, W. Haq, K. Srivastava, et al., Synthesis and antimalarial activity of side chain modified 4-aminoquinoline derivatives, J. Med. Chem. 50(2007) 394-398. |
| [6] | R.K. Rawal, R. Tripathi, S.B. Katti, et al., Design, synthesis and evaluation of 2-aryl-3-heteroaryl-1,3-thiazolidin-4-ones as anti-H(Ⅰ)V agents, Bioorg. Med. Chem. 15(2007) 1725-1731. |
| [7] | T. Shrivastava, A.K. Gaikwad, W. Haq, et al., Synthesis and biological evaluation of 4-thiazolidinone derivatives as potential antimicrobacterial agents, Arkivoc 2(2005) 120-130. |
| [8] | M.V. Diurno, O. Mazzoni, P.E. Calignano, et al., Synthesis and antihistaminic activity of some thiazolidin-4-ones, J. Med. Chem. 35(1992) 2910-2912. |
| [9] | N. Siddiqui, M.F. Arshad, S.A. Khan, et al., Sulfonamide derivatives of thiazolidin-4-ones with anticonvulsant activity against two seizure models:synthesis and pharmacological evaluation, J. Enzyme (Ⅰ)nhib. Med. Chem. 25(2010) 485-491. |
| [10] | C.M. Jackson, B. Blass, K. Coburn, et al., Evolution of thiazolidine-based blockers of human Kv1.5 for the treatment of atrial arrhythmias, Bioorg. Med. Chem. Lett. 17(2007) 282-284. |
| [11] | (a) T. Srivastava, W. Haq, S.B. Katti, Carbodiimide mediated synthesis of 4-thiazolidinones by one-pot three-component condensation, Tetrahedron 58(2002) 7619-7624;(b) A.C. Tripathi, S.J. Gupta, G.N. Fatima, et al., 4-Thiazolidinones:the advances continue, Eur. J. Med. Chem. 72(2014) 52-77. |
| [12] | R.C. Sharma, D. Kumar, Synthesis of some new thiazolidin-4-ones as possible antimicrobial agents, J. (Ⅰ)ndian Chem. Soc. 77(2000) 492-493. |
| [13] | T. Srivastava, W. Haq, S.B. Katti, Carbodiimide mediated synthesis of 4-thiazolidinones by one-pot three-component condensation, Tetrahedron 58(2002) 7619-7624. |
| [14] | R.K. Rawal, T. Srivastava, W. Haq, et al., An expeditious synthesis of thiazolidinones and tetathiazanones, J. Chem. Res. 5(2004) 368-369. |
| [15] | (a) C.P. Homes, J.P. Chinn, C.G. Look, et al., Strategies for combinatorial organic synthesis:solution and polymer-supported synthesis of 4-thiazolidinones and 4-metathiazanones derived from amino acids, J. Org. Chem. 60(1995) 7328-7333;(b) M.P. Thakare, P. Kumar, N. Kumar, et al., Silica gel promoted environmentfriendly synthesis of 2,3-disubstituted 4-thiazolidinones, Tetrahedron Lett. 55(2014) 2463-2466. |
| [16] | (a) A. Bolognese, G. Correale, M. Manfra, et al., Thiazolidin-4-one formation. Mechanistic and synthetic aspects of the reaction of imines and mercaptoacetic acid under microwave and conventional heating, Org. Biomol. Chem. 2(2004) 2809-2813;(b) A. Dandia, R. Singh, S. Bhaskaran, et al., Versatile three component procedure for combinatorial synthesis of biologically relevant scaffold spiro[indole-thiazolidinones] under aqueous conditions, Green Chem. 13(2011) 1852-1859;(c) D. Prasad, A. Pmreetam, M. Nath, DBSA catalyzed, one-pot three-component "on water" green protocol for the synthesis of 2,3-disubstituted 4-thiazolidinones, RSC Adv. 2(2012) 3133-3140;(d) D. Prasad, M. Nath, Three-component domino reaction in PPG:an easy access to 4-thiazolidinone derivatives, J. Heterocycl. Chem. 49(2012) 628-633. |
| [17] | (a) S.L. Xie, Y.H. Hui, X.J. Long, et al., Aza-Michael addition reactions between nitroolefins and benzotriazole catalyzedby MCM-41 immobilized heteropoly acids in water, Chin. Chem. Lett. 24(2013) 28-30;(b) F. Havasi, A. Ghorbani-Choqhamarani, F. Nikpour, Pd-grafted functionalized mesoporous MCM-41:a novel, green and heterogeneous nanocatalyst for the selective synthesis of phenols and anilines from aryl halides in water, New J. Chem. 39(2015) 6504-6512;(c) M. Nikoorazm, A. Ghorbani-Choqhamarani, H. Mahdavi, et al., Efficient oxidative coupling of thiols and oxidation of sulfides using UHP in the presence of Ni or Cd salen complexes immobilized on MCM-41 mesoporous as novel and recoverable nanocatalysts, Microporous Mesoporous Mater. 211(2015) 174-181;(d) A. Ghorbani-Choqhamarani, F. Nikpour, F. Ghorbani, et al., Anchoring of Pd(Ⅱ) complex in functionalized MCM-41 as an efficient and recoverable novel nano catalyst in C-C, C-O and C-N coupling reactions using Ph3SnCl, RSC Adv. 5(2015) 33212-33220. |
| [18] | X.Z. Dong, Y.H. Hui, S.L. Xie, et al., Schiff base supported MCM-41 catalyzed the Knoevenagel condensation in water, RSC Adv. 3(2013) 3222-3226. |
| [19] | (a) G.P. Zhou, L. Yu, Y.H. Hui, et al., Study on the epoxidation of α,β-unsaturated ketones catalyzed by MCM-41 supported Schiff base, Acta Chim. Sinica 70(2012) 1289-1294;(b) C.C. Wang, S.L. Xie, Z.F. Xie, et al., Michael addition reaction of malononitrile and α,β-unsaturated ketones catalyzed by amine functuonalized MCM-41, Chin. J. Org. Chem. 33(2013) 2391-2395;(c) K. Fan, Y.H. Hui, X.M. Hu, et al., PMoA/MCM-41 catalyzed aza-Michael reaction:special effects of mesoporous nanoreactor on chemical equilibrium and reaction rate through surface energy transformation, New J. Chem. 39(2015) 5916-5919. |
| [20] | D. Kumar, M. Sonawane, B. Pujala, et al., Supported protic acid-catalyzed synthesis of 2,3-disubstituted thiazolidin-4-ones:enhancement of the catalytic potential of protic acid by adsorption on solid supports, Green Chem. 15(2013) 2872-2884. |
 2016, Vol.27
2016, Vol.27