b. Shanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology, Shanghai 200237, China
Cysteine (Cys),homocysteine (Hcy),and glutathione (GSH) play a crucial role in many physiological and pathological processes,such as maintaining intracellular redox activities,xenobiotic metabolism,and gene regulation [1, 2]. Abnormal concentrations of these thiols are related to different diseases,including slowed growth in children,liver damage,skin lesions,Alzheimer’s disease,folate and cobalamin deficiencies,and cardiovascular diseases [3]. Many fluorescent and/or colorimetric probes have been developed for the selective detection of biothiols [4, 5]. Most of the reported probes showed similar responses toward different thiols. However,it is important to discriminate between biothiols because they exert distinct functions in biological systems [6].
Recently,some fluorescent probes for discrimination between GSH and Cys/Hcy were reported based on thiol-halogen nucleophilic substitution [5b,7]. The mercapto group in the biothiols replaced the halogen in the probes followed by the substitution of thiolate with amino in Cys/Hcy to form corresponding amino derivatives through five-/six-membered ring. The different photophysical properties of sulfur- and amino derivatives enable the distinction between GSH and Cys/Hcy.
NBD-F is widely employed as a derivation reagent for the detection of amino acids and proteins by HPLC [8]. The F group activated by the 7-nitro group can be easily substituted by nucleophilic reagents such as amino and mercapto groups to form the corresponding amino and thioether products. The absorption/ emission maxima of amino and thioether products are at about 470/=0 nm and 425/530 nm,respectively [6b,9]. SH is a better nucleophilic group than the amino group,however,the amino product is more stable. Therefore,thiols are expected to be discriminated form each other with NBD-F as the probe. Surfactant micelles have been used to promote chemical reactions and improve the detection sensitivity and selectivity of colorimetric/ fluorescent probes taking the advantage of the local concentration and the surface charge effects of micelles [10]. In this work,cetyltrimethylammonium bromide (CTAB) and sodium dodecyl sulfonate (SDS) were utilized as the detection media,the results revealed that the selectivity of NBD-F was improved in surfactant micelles.
2. ExperimentalUnless otherwise specified,all the reagents and solvents were of analytical grade and used without further purification. All the experiments were performed at 25 ℃ using non-degassed samples. NBD-F was purchased from TCI development Co.,Ltd (Shanghai). Ultra-pure water (18.2 MV cm) was prepared through a Sartorius Arium611DI system. Absorption spectra were measured on a Varian Cary 50 spectrophotometer and emission spectra were recorded on a Varian Cary Eclipse fluorescence spectrophotometer. 18 μL of freshly-prepared NBD-F stock solution (5 mmol/L,in acetonitrile solution) were dissolved in 3 mL of corresponding solvent to keep [NBD-F] = 30 μmol/L. 10 μL of analyte solution (9 mmol/L) were added to the above solution to keep [analyte] = 30 μmol/L. Absorption and fluorescence spectra were collected at different time intervals.
3. Results and discussion 3.1. The spectral response of NBD-F toward ME in different mediumWe first selected mercaptoethanol (ME) as the model of thiols to study the reaction activity of NBD-F in different reaction media. Fig. S1A (Supporting information) displays the spectral response of NBD-F toward ME in Tris-HCl buffer (pH 7.4). Upon addition of ME,the absorption band of NBD-F centered at 335 nm decreased gradually; meanwhile,a new peak at 425 nm emerged and developed steadily with a well-defined isosbestic point at 360 nm. The absorption peak at 430 nm could be ascribed to the mercapto-substituent of NBD-F. In CTAB and SDS micelles,similar but faster spectral responses of NBD-F are obtained (Fig. S1A and B in Supporting information).
Fig. 1 is the time-dependent absorbance ratios of At/A0 at 335 nm and 425 nm of NBD-F-ME in different media. It is clear that the reaction between NBD-F and ME was very slow and was not completed after 2 h in Tris-HCl buffer. However,the reaction is much faster in micelles,and it was finished within 60 min and 10 min in SDS and CTAB micelles,respectively. Surfactants affect the reaction in two ways: (1) they provide a hydrophobic microenvironment for the organic reactants,which enhances the solubility and the local concentrations of the reactants,as a result,the reaction is accelerated dramatically; (2) they change the local OH- concentration around micelles. The substitution of F by SH is a nucleophilic reaction,which is accelerated by base (Fig. S2). Compared to the bulk solution,the OH- concentration on cationic surfactant CTAB micelle surface is much higher,while that on anion surfactant SDS micelle surface is much lower. Therefore,the reaction rate in CTAB micelles is the highest.

|
Download:
|
| Fig. 1.Time-dependent absorbance ratios of At/A0 at 335 nm (a) and 425 nm (b) of NBD-F-ME in different media. [SDS] = [CTAB] = 5 mM,[NBD-F] = [ME] = 30 mM,25 ℃,25 mM Tris-HCl (pH 7.4). | |
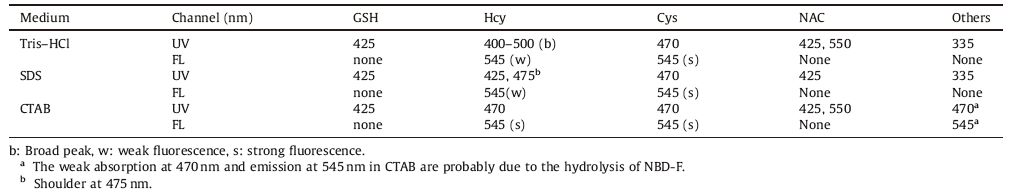
(pH 7.4) The spectral responses of NBD-F toward different analytes in Tris-HCl buffer (pH 7.4) were investigated (Fig. 2). GSH,ME,and mercaptoacetic acid (MPA) made the original peak at 335 nm decrease,and a new peak at about 425 nm emerged,whereas Cys induced the appearance of a new absorption peak at 470 nm. In the case of Hcy,a broad band ranged from 400 to 500 nm was observed. Two new peaks at 425 nm and 530 nm formed with the addition of N-acetyl-L-cysteine (NAC). With 470 nm as the excitation wavelength,Cys triggered about a 10-fold fluorescence enhancement at 545 nm. Other additives except for Hcy did not induce obvious fluorescence changes. The absorption at 470 nm and emission at 545 nm correspond to the amino substitute,and the absorption at 430 nm could be ascribed to the thiolether derivate of NBD-F. Fig. 2d shows the fluorescence intensity at 545 nm of NBD-F in the presence of various additives,from which it is clear that NBD-F is highly selective toward Cys in Tris-HCl buffer. Therefore,NAC and Cys can be distinguished from other thiols and amino acids by the absorption and emission spectra,respectively.

|
Download:
|
| Fig. 2.The absorption (a and b) and emission (c) spectra of NBD-F toward different analytes in 25 mMTris-HCl buffer (pH 7.4) and the corresponding fluorescence intensity at 545 nm(d). (1) blank; (2) Cys; (3) Hcy; (4) Ile; (5) GSH; (6) NAC; (7) ME; (8) MPA; (9) n-butylamine (BA); (10) Gly; (11) His; (12) Met; (13) Tyr; (14) His; (15) Gla; (16) Trp; (17) Val; (18) Ala; (19) Asp; (20) Phe; (21) Ser. [NBD-F] = [analyte] = 30 μM,equilibrated at 25 ℃ for 30 min,bda;ex = 470 nm. | |
Fig. 3 is the UV-vis spectra of NBD-F in the presence of different analytes in SDS micelles. The thiols,including GSH/MPA/ME and NAC initiate a new absorption peak at -425 nm (Fig. 3a). The presence of Cys led to the appearance of a new peak at 477 nm. Hcy induced the emergence of an absorption band at 425 nm with a shoulder peak at -480 nm (Fig. 3b). No obvious spectral changes were observed with the addition of other amino acids (Fig. 3a). With 450 nm as the excitation wavelength,the fluorescence emission spectral responses were similar to those in Tris-HCl buffer (Fig. S4). According to the changes in the absorption and emission spectra,Cys could be discriminated from other thiols and amino acids.

|
Download:
|
| Fig. 3.The absorption spectra of NBD-F in the presence of different additives in 5 mmol/L SDS micelles. Other analytes including Try,Asp,Val,His,Gly,Met,Gla,Ile,Trp,Ser,Phe,Ala and Tyr. [NBD-F] = [analyte] = 30 mmol/L,equilibrated at 25 ℃ for 30 min in Tris-HCl buffer (25 mmol/L,pH 7.4) containing 5 mmol/LSDS. | |
The spectral responses of NBD-F toward various analytes in CTAB micelles were studied as well. It is interesting to note that the presence of Cys led to the appearance of a new peak at 470 nm which reached its maximum within 2 min and then decreased,accompanied by the emergence of an absorption peak at =0 nm (Fig. 4a). The solution’s color changed from yellow to pink. With the addition of NAC,the original absorption band decreased,accompanied by two new peaks at 425 nm and =0 nm (Fig. 4a). Similar to that in Tris-HCl buffer and SDS micelles,the addition of GSH and ME caused about 90 nm red-shifts of the absorption spectrum of NBD-F (Fig. 4b). The presence of Hcy made the absorption peak shift from 335 nm to 470 nm (Fig. 4c). Other analytes did not induce obvious spectral changes (Fig. 4c).

|
Download:
|
| Fig. 4.The absorption spectra of NBD-F in the presence of different additives in 5 mmol/L CTAB-Tris-HCl (25 mM,pH = 7.4),[NBD-F] = [analyte] = 30 mmol/L,equilibrated at 25 ℃ for 10 min. | |
The addition of Cys to NBD-F solution made the fluorescence intensity at 545 nm increase first and then decrease,revealing that the product with lab = =0 nm is non-fluorescent (Fig. S3 in Supporting information). Other additives could interfere with the detection of Cys (Fig. S5 in Supporting information),therefore,CTAB is not a good medium for selective detection of Cys/Hcy. The effects of different analytes on the spectra of NBD-F are summarized in Table 1. From which,we can distinguish GSH,Hcy,Cys and NAC from each other.
|
|
Table 1 The spectral response of NBD-F toward various thiols. |
From the above results,we know that Cys formed aminosubstitute,while GSH formed thioether-substitute with NBD-F in all three media. As for Hcy,thiolether and amino substitutes coexisted in Tris-HCl and SDS micelles,whereas amino substitute was the main product in CTAB micelles. The OH- concentration on CTAB micelle surface is much higher,which increased the reaction rate to give more amino derivates.
The sensing mechanism of NBD-F toward thiols is similar to the reported works [11,5e],which includes two step reactions: (1) the mercapto group in thiol substituted the F group in NBD-F followed by (2) the replacement of alkylthiol by amino group through intramolecular nucleophilic aromatic substitution via a multiplering transition state to form amino-derivates (Scheme 1). The stability of the multiple-rings decreases in the order of 5-membered > 6-membered > 10-membered. In addition,the local OH- concentrations in the three media are different; therefore,NBD-F exhibited different spectral responses toward thiols. It is interesting to note that a new absorption peak at =0 nm was formed with the addition of NAC to NBD-F in CTAB micelles and Tris-HCl media,the much longer wavelength may be caused by the π-π stacking between electron-deficient fluorophore and the electron-rich carboxyl group in basic solutions [12]. Similarly,Cys could form π-π stacking state in CTAB micelles to some extent,which made NAC and Cys be discriminated from other thiols.

|
Download:
|
| Scheme 1.The proposed reaction mechanism of NBD-F with GSH/Cys/Hcy/NAC. | |
NBD-F,an easily-obtained commercial reagent,was employed as a fluorescent and colorimetric probe for thiols. The selectivity and the spectral response of the probe were improved by elaborately selecting a proper detection medium. Surfactant micelles can speed up the detection of thiols by providing hydrophobic microenvironments. This work may provide a new strategy for improving the properties of known probes by choosing an appropriate detection medium.
AcknowledgmentsThis work was financially supported by Shanghai Municipal Natural Science Foundation(No.15ZR1409000) andtheopen fundof Shanghai Key Laboratory of Chemical Biology (No. SKLCB-2013-03).
| [1] | (a) S. Zhang, C.N. Ong, H.M. Shen, Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells, Cancer Lett. 208(2004) 143-153;(b) D.M. Townsend, K.D. Tew, H. Tapiero, Sulfur containing amino acids and human disease, Biomed. Pharmacother. 58(2004) 47-55;(c) H. Tapiero, D.M. Townsend, K.D. Tew, The antioxidant role of selenium and seleno-compounds, Biomed. Pharmacother. 57(2003) 134-144;(d) Z.A. Wood, E. Schröder, J.R. Harris, L.B. Poole, Structure, mechanism and regulation of peroxiredoxins, Trends Biochem. Sci. 28(2003) 32-40. |
| [2] | (a) C. Hwang, A.J. Sinskey, H.F. Lodish, Oxidized redox state of glutathione in the endoplasmic reticulum, Science 257(1992) 1496-1502;(b) A. Meister, Glutathione metabolism and its selective modification, J. Biol. Chem. 263(1988) 17205-17208;(c) T.P. Dalton, H.G. Shertzer, A. Puga, Regulation of gene expression by reactive oxygen, Annu. Rev. Pharmacol. Toxicol. 39(1999) 67-101;(d) L.A. Herzenberg, S.C. De Rosa, J.G. Dubs, et al., Glutathione deficiency is associated with impaired survival in H(Ⅰ)V disease, Proc. Natl. Acad. Sci. U.S.A. 94(1997) 1967-1972;(e) C. Perricone, C. De Carolis, R. Perricone, Glutathione:a key player in autoimmunity, Autoimmun. Rev. 8(2009) 697-701. |
| [3] | (a) L. El-Khairy, P.M. Ueland, H. Refsum, (Ⅰ).M. Graham, S.E. Vollset, Plasma total cysteine as a risk factor for vascular disease:the European concerted action project, Circulation 103(2001) 2544-2549;(b) S. Shahrokhian, Lead phthalocyanine as a selective carrier for preparation of a cysteine-selective electrode, Anal. Chem. 73(2001) 5972-5978;(c) S. Seshadri, A. Beiser, J. Selhub, et al., Plasma homocysteine as a risk factor for dementia and Alzheimer's disease, N. Engl. J. Med. 346(2002) 476-483;(d) H. Refsum, A.D. Smith, P.M. Ueland, et al., Facts and recommendations about total homocysteine determinations:an expert opinion, Clin. Chem. 50(2004) 3-32. |
| [4] | (a) X. Chen, Y. Zhou, X. Peng, J. Yoon, Fluorescent and colorimetric probes for detection of thiols, Chem. Soc. Rev. 39(2010) 2120-2135;(b) H.S. Jung, X. Chen, J.S. Kim, J. Yoon, Recent progress in luminescent and colorimetric chemosensors for detection of thiols, Chem. Soc. Rev. 42(2013) 6019-6031. |
| [5] | (a) Y. Liu, X. Lv, J. Liu, et al., Construction of a selective fluorescent probe for GSH based on a chloro-functionalized coumarin-enone dye platform, Chem. Eur. J. 21(2015) 4747-4754;(b) X.D. Zeng, X.L. Zhang, B.C. Zhu, et al., A highly selective wavelength-ratiometric and colorimetric probe for cysteine, Dyes Pigm. 94(2012) 10-15;(c) B.X. Zhang, C.P. Ge, J. Yao, et al., Selective selenol fluorescent probes:design, synthesis, structural determinants, and biological applications, J. Am. Chem. Soc. 137(2015) 757-769;(d) L. Yang, W.S. Qu, X. Zhang, et al., Constructing a FRET-based molecular chemodosimeter for cysteine over homocysteine and glutathione by naphthalimide and phenazine derivatives, Analyst 140(2015) 182-189;(e) L. Song, T. Jia, W.J. Lu, et al., Multi-channel colorimetric and fluorescent probes for differentiating between cysteine and glutathione/homocysteine, Org. Biomol. Chem. 12(2014) 8422-8427;(f) Y.H. Li, J.F. Yang, C.H. Liu, et al., Colorimetric and fluorescent detection of biological thiols in aqueous solution, Chin. Chem. Lett. 24(2013) 96-98. |
| [6] | (a) M. (Ⅰ)şık, R. Guliyev, S. Kolemen, et al., Designing an intracellular fluorescent probe for glutathione:two modulation sites for selective signal transduction, Org. Lett. 16(2014) 3260-3263;(b) D. Lee, G. Kim, J. Yin, J. Yoon, An aryl-thioether substituted nitrobenzothiadiazole probe for the selective detection of cysteine and homocysteine, Chem. Commun. 51(2015) 6518-6520;(c) L. Wang, H.Y. Chen, H.L. Wang, et al., A fluorescent probe with high selectivity to glutathione over cysteine and homocysteine based on positive effect of carboxyl on nucleophilic substitution in CTAB, Sens. Actuators, B:Chem. 192(2014) 708-713;(d) J. Liu, Y.Q. Sun, Y.Y. Huo, et al., Simultaneous fluorescence sensing of Cys and GSH from different emission channels, J. Am. Chem. Soc. 136(2014) 574-577. |
| [7] | (a) L.Y. Niu, Y.S. Guan, Y.Z. Chen, et al., BOD(Ⅰ)PY-based ratiometric fluorescent sensor for highly selective detection of glutathione over cysteine and homocysteine, J. Am. Chem. Soc. 134(2012) 18928-18931;(b) L.Y. Niu, H.R. Zheng, Y.Z. Chen, et al., Fluorescent sensors for selective detection of thiols:expanding the intramolecular displacement based mechanism to new chromophores, Analyst 139(2014) 1389-1395;(c) L.A. Montoya, M.D. Pluth, Hydrogen sulfide deactivates common nitrobenzofurazan-based fluorescent thiol labeling reagents, Anal. Chem. 86(2014) 6032-6039;(d) Y.H. Chen, J.C. Tsai, T.H. Cheng, et al., Sensitivity evaluation of NBD-SCN towards cysteine/homocysteine and its bioimaging applications, Biosens. Bioelectron. 56(2014) 117-123. |
| [8] | (a) Y. Watanabe, K. (Ⅰ)mai, Pre-column labelling for high-performance liquid chromatography of amino acids with 7-fluoro-4-nitrobenzo-2-oxa-13-diazole and its application to protein hydrolysates, J. Chromatogr., A 239(1982) 723-732;(b) T. (Ⅰ)shikawa, H. (Ⅰ)mai, K.Y. Maki, Development of an LC-MS/MS method for the analysis of free sphingoid bases using 4-fluoro-7-nitrobenzofurazan (NBD-F), Lipids 49(2014) 295-304;(c) X.M. Wu, R. Wang, Q.Q. Jiang, et al., Determination of amino acid neurotransmitters in rat hippocampi by HPLC-UV using NBD-F as a derivative, Biomed. Chromatogr. 28(2014) 459-462;(d) Y. Song, T. Funatsu, M. Tsunoda, Amino acids analysis using a monolithic silica column after derivatization with 4-fluoro-7-nitro-21,3-benzoxadiazole (NBD-F), J. Chromatogr. B:Analyt. Technol. Biomed. Life Sci. 879(2011) 335-340. |
| [9] | D.J. Birkett, N.C. Price, G.K. Radda, A.G. Salmon, The reactivity of SH groups with a fluorogenic reagent, FEBS Lett. 6(1970) 346-348. |
| [10] | (a) S. Uchiyama, K. (Ⅰ)wai, A.P. de Silva, Multiplexing sensory molecules map protons near micellar membranes, Angew. Chem. (Ⅰ)nt. Ed. 47(2008) 4667-4669;(b) J.H. Qian, S.H. Qian, R. Guo, The effects of anionic and cationic surfactants on the hydrolysis of sodium barbital, J. Surfactants Deterg. 8(2005) 253-256;(c) Y.X. Guo, X.F. Yang, L.H. Hakuna, et al., A fast response highly selective probe for the detection of glutathione in human blood plasma, Sensors 12(2012) 5940-5950;(d) H.Y. Tian, J.H. Qian, H.Y. Bai, et al., Micelle-induced multiple performance improvement of fluorescent probes for H2S detection, Anal. Chim. Acta 768(2013) 136-142;(e) L. Song, H.Y. Tian, X.L. Pei, et al., Colorimetric and fluorescent detection of GSH with the assistance of CTAB micelles, RSC Adv. 5(2015) 59056-59061. |
| [11] | L.M. Ma, J.H. Qian, H.Y. Tian, et al., A colorimetric and fluorescent dual probe for specific detection of cysteine based on intramolecular nucleophilic aromatic substitution, Analyst 137(2012) 5046-5050. |
| [12] | (a) Y.Y. Chen, L.P. Si, J.J. Liu, et al., Study on π-π stacking interaction of aryl and alkyl meso-substituted corroles and theirs copper complexes, Comput. Appl. Chem. 26(2009) 1587-1592;(b) B.N. Li, Y.K. Wang, D.M. Du, J.X. Xu, Notable and obvious ketene substituentdependent effect of temperature on the stereoselectivity in the Staudinger reaction, J. Org. Chem. 72(2007) 990-997. |
 2016, Vol.27
2016, Vol.27