b. State Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China
In the design of new solid-state crystalline materials,hybrid inorganic-organic coordination polymers are of great interest due to their rational assembly by virtue of the judicious selection of inorganic moieties and organic linkers [1, 2, 3, 4, 5]. Among such materials,those uranium-bearing have attracted increasing attentions in recent years because of their intriguing architectures and valuable properties [6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16]. As the main component in nuclear fuel,uranium exhibits extraordinary fission ability that is the key feature for use in manufacturing of nuclear weapons and the generation of electricity. In general,elemental uranium exists with four oxidation states from +3 up to +6. Among them,hexavalent uranium,which forms a specific configuration of linear uranyl (UO2 2+) cation,is of particular interest not only because of the stability feature of U(VI) under ambient atmosphere,but also due to the critical relevance to the long term storage of nuclear fuels. So far a rich variety of uranyl-organic hybrid materials including molecular complexes and extended multi-dimensional network structures (1D,2D and 3D) are known [7]. The investigation of uranyl-organic assemblies not only can reveal synergistic interaction between uranium and organic ligands from the structural perspective,more importantly,can provide an implicit relationship among studies of synthesis,speciation and complexation of uranyl materials.
Except for the varied polynuclear uranyl species polymerized by the three coordination polyhedra (square,pentagonal and hexagonal bipyramids),the key to constructing uranyl-organic assemblies with novel structures and outstanding features mainly depends on the intrinsic characteristic of the organic ligand,whose acidity,flexibility,dimensions and steric effect can greatly influence the molecular coordination modes and packing arrangements of the resultant complex. Compared with other uranyl- organic hybrids,carboxylates have been intensively studied including rigid aromatic and flexible aliphatic species. Recent reviews dedicated by Loiseau and Chen give comprehensive presentations in terms of structures and properties [6, 7]. Among the various carboxylate ligands that were adopted to isolate uranyl materials,rigid-flexible mixed polycarboxylic acids are very attractive for the potential to construct uranyl-organic structures with novel architectures by virtue of both the characters of rigid and flexible components [17, 18]. More recently,we reported a series of uranyl polymeric structures by utilizing 4,4'-oxidiphthalic acid (H4L) as the ligand [19]. Its inherent chemical features are follows: (1) the V-shaped tetracarboxylate ligand with two chemically robust aromatic rings around the center oxygen atom exhibits flexibility; (2) the carboxylate groups with classical O-donor sites exhibit strong affinity for binding uranium centers; (3) four carboxylate moieties can be completely or partially deprotonated,that can lead to rich coordination modes for ligating metal centers. Meanwhile the protonated or deprotonated carboxylate functions can serve as hydrogen-bond donors or acceptors to interact with organic templates or solvent molecules,thereby forming supra-molecular structures or further strengthening the framework stability. As an ongoing work,three uranyl compounds of 4,4'-oxidiphthalic acid were isolated in this study,namely,(NH4)2[(UO2)3(L)2]-5H2O (1),(NEt4)[(UO2)3(H2O)(L)(HL)] (2) and (UO2)7(H2O)2(phen)4(L)2(HL)2 (3) (NEt4 = tetraethylammonium,phen = 1,1'-phenanthroline). Herein,we report on their syntheses,structures and properties.
2. ExperimentalCaution! Standard procedures for handling radioactive material should be followed,although the uranyl compounds used in the lab contained depleted uranium.
2.1. Materials and methodsAll chemicals were purchased commercially and used without further purification. Elemental analyses of C,H,and N were conducted on a Perkin-Elmer 2400 elemental analyzer. Thermogravimetric analysis (TGA) was made using a SDT 2960 Simultaneous DSC-TGA of TA instruments up to 800 ℃,and the heating rate was 10 ℃/min under an air flow of 100 mL/min. Infrared spectra were collected from single crystals of all the uranyl complexes using a Nicolet 6700 FT-IR spectrometer. The spectra were collected with a diamond ATR objective. Powder X-ray diffraction (PXRD) patterns were performed on a D8 Focus (Bruker) diffractometer with Cu-Ka radiation Field-emission (l = 1.5405Å ,continuous,40 kV,40 mA,increment = 0.028). The photoluminescence (PL) excitation and emission spectra were recorded with F-7000 luminescence spectrometer equipped with a Xenon lamp of 450W as an excitation light source. The photomultiplier tube voltage was 400 V,the scan speed was 1200 nm/min,both the excitation and the emission slit width were 5.0 nm.
2.2. SynthesesA mixture of UO2(NO3)2-6H2O (50 mg,0.1 mmol),4,40-oxidiphthalic acid (35 mg,0.1 mmol),NH3-H2O (50 mL) for 1,NEt4OH (25%,30 mL) and HNO3 (65%,20 mL) for 2,or phen (18 mg,0.1 mmol) for 3 as well as deionized water (1.0 mL) was loaded into a 20-mL Teflon-lined stainless steel autoclave. The autoclave was sealed and heated at 160 ℃ for 3 days,and then cooled to room temperature. Yellow block crystals suitable for single X-ray diffraction analysis were isolated. Yields are 19 mg (35%) for 2 and 27 mg (47%) for 3 based on uranium. PXRD patterns of 2 and 3 shown in Figs. S1 and S2 in Supporting information prove the phase purity. Elemental analysis observed (calcd.): C 29.86% (29.23%); H 2.13% (2.15%); N 0.90% (0.85%) for 2. C 33.39% (33.48%); H 1.57% (1.=%); N 2.83% (2.79%) for 3. 1 is the minor phase combined with major unidentified powder. Thermogravimetric analysis (TGA) studies reveal that 2 and 3 can be stable to 350 ℃ and 400 ℃,respectively,after that the networks start collapsing upon further calcination (Fig. S3 in Supporting information). The final product may be U3O8 (exp. (calcd.): 50.3% (51.2%) for 2,45.3% (46.9%) for 3).
2.3. X-ray crystal structure determinationSuitable single crystals for title compounds were selected for single-crystal X-ray diffraction analyses. Crystallographic data were collected at 296 K on a Bruker Apex Ⅱ CCD diffractometer with graphite monochromated Mo-Kα radiation (bda; = 0.71073Å ). Data processing was accomplished with the SAINT program [20]. The structures were solved by direct methods and refined on F2 by full-matrix least squares using SHELXTL-97 [21]. Non-hydrogen atoms were refined with anisotropic displacement parameters during the final cycles. All hydrogen atoms were placed by geometrical considerations and were added to the structure factor calculation. A summary of the crystallographic data for these title complexes is listed in Table 1. Selected bond distances and angles are given in Tables S1-3 in Supporting information. Further details of the crystal structure investigation may be obtained from the Cambridge Crystallographic Data Centre on quoting numbers CCDC 1052270-1052272.
|
|
Table 1 Crystal data and structural refinement for title compounds. |
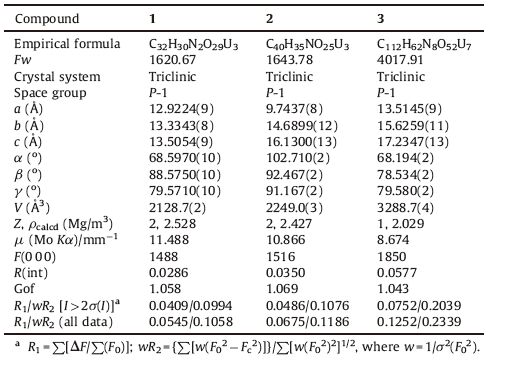
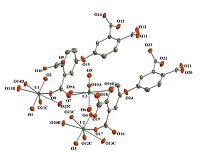
All the three uranyl carboxylates crystallize in the triclinic space group P-1. The asymmetric unit of 1 consists of three crystallographically unique uranium atoms,two L ligands,two NH4 + ions and five lattice water molecules. As shown in Fig. 1,all the U atoms are in the similar coordination environment of being seven coordinated by two axial oxo atoms and five equatorial O atoms from four L ligands. Thus common pentagonal bipyramids are formed. The U=O distances in the axis range from 1.758(7) to 1.775(8)Å ,and the U-O bond lengths in the equatorial plane are in the range of 2.341(6)-2.574(7)Å . These values are typical for U(VI)-bearing compounds. The calculated bond-valence sum based on these values indicates 6.15 for U(1),6.14 for U(2) and 5.96 for U(3),which are consistent with the formal valence of U(VI) [22, 23]. The uranyl pentagonal bipyramids are linked by the tetracarboxylate ligands creating a 3D framework structure,which features 1D rectangular channels parallel to b axis (Fig. 2). This channel is formed by four uranyl polyhedra and four ligands with the internal dimensions of approximately 5.9 ± 11.9Å (including Van der Waals radius). NH4 ions and water molecules are involved in these channels and interact with the skeleton via hydrogen bonds (D-H- × -O: 2.487-2.799Å ).
|
Download:
|
| Fig. 1.The coordination environment in 1. Displacement ellipsoids are drawn at the 50% probability level. Symmetry codes: (A),1 × x,2 × y,2 × z; (B) x,y,1 + z; (C) -x,1 × y,2 × z; (D) -x,2 × y,2 × z; (E) 1 × x,1 × y,2 × z. | |
|
Download:
|
| Fig. 2.The framework structure of 1 with NH4 ions and water molecules in the channels. | |
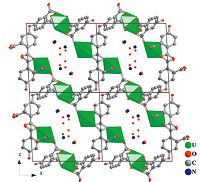
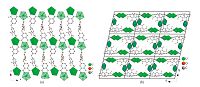
The asymmetric unit of 2 also contains three crystallographically unique uranium centers in pentagonal bipyramidal environments (Fig. 3). Five carboxylate oxygen atoms from four ligands are coordinated to U(1) and U(2) perpendicular to the uranyl axis. For U(3),four oxygen atoms from three ligands and one coordinated water molecule are arranged in the equatorial plane. The equatorial U-O bond lengths range from 2.328(9) to 2.452(7)Å ,which are common for uranyl materials. Bond valence sum gives the calculated values of 6.12 for U(1),6.16 for U(2) and 6.30 for U(3). The U(1) and U(2) centers are bridged by organic ligands into a single layered arrangement (Fig. 4a). Such layers are further linked by U(3)-centered polyhedra through the carboxylate groups,generating a double layered architecture. As shown in Fig. 4b,there are channels within the double layered structure that run parallel to the a axis. NEt4 + cations serve as counter-ions locating in the channels.
|
Download:
|
| Fig. 3.The coordination environment in 2. Displacement ellipsoids are drawn at the 50% probability level. Symmetry codes: (A) -1 + x,y,z; (B) -x,1 × y,2 × z; (C) x,y,-1 + z; (D) -x,1 × y,1 × z; (E) -1 + x,y,1 + z. | |
|
Download:
|
| Fig. 4.(a) The single layer isolated from 2. U(1) and U(2) centered pentagonal bipyramids are shown in closed and translucent polyhedra,respectively. (b) The double layers pack forming the whole structure. The connected U(3)-centered pentagonal bipyramids are highlight in blue border. The NEt4 + cations are removed for clarity. | |
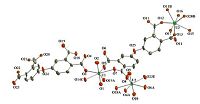
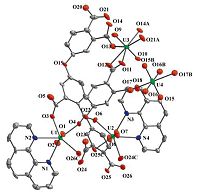
There are four crystallographically unique uranium atoms,two L ligands and two phen molecules in the asymmetric unit of 3 (Fig. 5). The coordination spheres of U(1) and U(2) are similar,which are defined by two axial oxo atoms,three equatorial oxygen atoms from two ligands and two N atoms from one phen molecule. The average U=O distances are 1.745(12)Å for U(1) and 1.746(13)Å for U(2). The equatorially coordinated oxygen atoms are at distances of 2.282(12)-2.334(12)Å from U centers,and the U-N bond lengths are slightly longer with 2.580(15)-2.676(15)Å . U(1) and U(2) centered polyhedra are bridged by two carboxylate moieties into a dimer. U(3) is in a pentagonal bipyramidal environment defined by two uranyl oxygen atoms (U=O: 1.740(12) and 1.769(13)Å ) and five equatorial oxygen atoms from three ligands (U-O: 2.292(15)-2.439(12)Å ). Connection of two U(3)-centered pentagonal bipyramids by two carboxylate groups leads to another dimer. Such dimers together with U(1) and U(2) dimers are linked by L ligands,forming 1D arrangements,which are further bridged by U(4) centers to generate the layered structure of 3 (Fig. 6a). U(4) is six-fold coordinated by two axial oxygen atoms,two carboxylate oxygen atoms from two ligands and two terminal oxygen atoms in the equatorial plane. The axial U=O distances are 1.7=(17)Å ,two shorter U-O distances are 2.35(2)Å ,and two longer U-O bond lengths of 2.40(2)Å are also terminal,thus are assigned as coordinated water molecules. The calculated bond-valence sum indicates 6.10 for U(1),6.11 for U(2),6.19 for U(3) and 5.67 for U(4). A view of the whole structure along c axis shows the packing of the layers (Fig. 6b),which interact with each other via p-p stacking of the coordinated phen molecules (the inter-centroid distances of 3.66-3.94Å ).
|
Download:
|
| Fig. 5.The coordination environment in 3. Displacement ellipsoids are drawn at the 50% probability level. Symmetry codes: (A) 1 × x,2 × y,1 × z; (B) 1 × x,2 × y,-z; (C) 2 × x,1 × y,-z. | |

|
Download:
|
| Fig. 6.(a) The individual layered structure of 3. Six-fold U(4) centers are highlight with dark green. Phen molecules are deleted for clarity. (b) Such layers interact via p-p stacking. Individual layers are indicated by different colors for clarity. | |
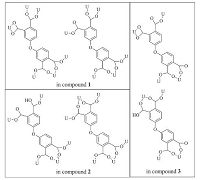
The tetracarboxylate ligand displays flexible coordination fashions for binding uranyl centers in these uranyl assemblies,which are summarized in Scheme 1. In 1,both of the two unique ligands are completely deprotonated and serve as hexadentate connectors,linking the nearby six U centers with different coordination manners,one in μ6 (η2: η1: η1: η1: η2: η1) and the other in μ6 (η1: η1: η1: η1: η2: η1). In 2,one unique ligand is incompletely deprotonated with one carboxylate group being protonated. This ligand adopts μ5 (η1: η1: η1: η2: η1) coordination model,linking five U centers. The other completely deprotonated ligand takesμ6 (η1: η2: η1: η1: η2: η1) coordination models,linking six U centers. It is interesting that this μ6-brigding model is different from that in 1. While in 3,two incompletely and completely deprotonated ligands serve as different types ofμ4 (η2: η1: η2: η1) coordination modes,linking four uranyl centers. Notably,these coordination modes are different from that observed in reported uranyl compounds of 4,40-oxidiphthalic acid [19]. The variation and richness of coordination manners of this ligand are further demonstrated in this work.
|
Download:
|
| Scheme 1.Summary of coordination modes of 4,40-oxydiphthalic acid in title uranyl compounds. | |
IR spectra of these synthesized uranyl carboxylates are displayed in Fig. S4 in Supporting information. The characteristic vibrations of UO2 2+ cations are displayed around 918 and 846 cm-1,which are attributed to the symmetrical n1 and antisymmetric elongations n3,respectively [24, 25]. The vibrations of carboxylate groups and the aromatic rings are indicated in the bands of 1640-1100 cm-1.
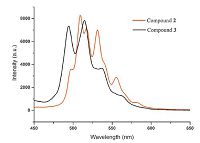
3.3. Luminescent propertyIn general,uranyl-bearing compounds can emit green light centered near 520 nm that is based on the charge-transfer emission. The spectrum always consists of several emission peaks [26]. Such behavior is in fact related to both the bending and stretching vibrational modes of the uranyl cation. As depicted in Fig. 7,multiple peaks ranging from 494 nm to 564 nm are clearly resolved in the emission spectra of 2 and 3,that are ascribed to the electronic and vibronic transitions S11-S00 and S10-S0v (v = 0-4). The minor difference between the two spectra is probably due to the different uranyl coordination environments,UO6 square bipyramid,UO7 and UO5N2 pentagonal bipyramids,as well as the effects of the carboxylate ligand and N-species.
|
Download:
|
| Fig. 7.The luminescent spectra of 2 and 3 showing the uranyl emission of green light. | |
By employing 4,40-oxidiphthalic acid as the ligand,we have successfully synthesized three new uranyl-organic assemblies including one 3D and two 2D structures. In these structures,the carboxylate ligands serve as tetradentate,pentadentate and hexadentate linkers with different coordination manners for binding uranium centers. Different N-species assist the formation of these compounds. In 1 and 2,NH4 + and NEt4 + ions are accommodated in the channels of their networks,respectively,acting as counter-ions. Whereas in 3,phen molecules coordinate to uranyl centers as the coligands to participate in the network. Photoluminescent studies reveal both 2 and 3 are fluorescent with green light emission of uranyl cation.
AcknowledgmentsWe thank the support of this work by National Natural Science Foundation of China (Nos. 21571171,21301168,U1407101) and Jilin Province Youth Foundation (No. 20130522123JH). Appendix A. Supplementary data Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.12.016.
| [1] | T.R. Cook, Y.R. Zheng, P.J. Stang, Metal-organic frameworks and self-assembled supramolecular coordination complexes:comparing and contrasting the design, synthesis, and functionality of metal-organic materials, Chem. Rev. 113(2013) 734-777. |
| [2] | J. Park, D.W. Feng, H.C. Zhou, Structure-assisted functional anchor implantation in robust metal-organic frameworks with ultralarge pores, J. Am. Chem. Soc. 137(2015) 1663-1672. |
| [3] | W. Wang, Y. Yuan, F.X. Sun, G.S. Zhu, Targeted synthesis of novel porous aromatic frameworks with selective separation of CO2/CH4 and CO2/N2, Chin. Chem. Lett. 25(2014) 1407-1410. |
| [4] | T. Zheng, M. Ren, S.S. Bao, L.M. Zheng, M2(pbtcH)(phen)2(H2O)2[M(Ⅱ)=Co, Ni]:mixed-ligated metal phosphonates based on 5-phosphonatophenyl-1,2,4-tricarboxylic acid showing double chain structures, Chin. Chem. Lett. 25(2014) 835-838. |
| [5] | Y.X. Sun, W.Y. Sun, (Ⅰ)nfluence of temperature on metal-organic frameworks, Chin. Chem. Lett. 25(2014) 823-828. |
| [6] | K.X. Wang, J.S. Chen, Extended structures and physicochemical properties of uranyl-organic compounds, Acc. Chem. Res. 44(2011) 531-540. |
| [7] | T. Loiseau, (Ⅰ). Mihalcea, N. Henry, C. Volkringer, The crystal chemistry of uranium carboxylates, Coord. Chem. Rev. 266-267(2014) 69-109. |
| [8] | K.E. Knope, C.L. Cahill, Uranyl phosphonates:a structural survey, in:A. Clearfield, K. Demadis (Eds.), Metal Phosphonate Chemistry:From Synthesis to Applications, The Royal Society of Chemistry, London, 2012, pp. 586-606. |
| [9] | Z.H. Weng, Z.H. Zhang, T. Olds, M. Sterniczuk, P.C. Burns, Copper((Ⅰ)) and copper(Ⅱ) uranyl heterometallic hybrid materials, (Ⅰ)norg. Chem. 53(2014) 7993-7998. |
| [10] | P.O. Adelani, N.D. Cook, P.C. Burns, Use of 2,2-bipyrimidine for the preparation of UO22+-3d diphosphonates, Cryst. Growth Des. 14(2014) 5692-5699. |
| [11] | P. Thuéry, J. Harrowfield, Uranyl-organic frameworks with polycarboxylates:unusual effects of a coordinating solvent, Cryst. Growth Des. 14(2014) 1314-1323. |
| [12] | P. Thuery, J. Harrowfield, Chiral one-to three-dimensional uranyl-organic assemblies from (1R,3S)-(+)-camphoric acid, CrystEngComm 16(2014) 2996-3004. |
| [13] | T.G. Parker, J.N. Cross, M.J. Polinski, J. Lin, T.E. Albrecht-Schmitt, (Ⅰ)onothermal and hydrothermal flux syntheses of five new uranyl phosphonates, Cryst. Growth Des. 14(2014) 228-235. |
| [14] | W.T. Yang, T.G. Parker, Z.M. Sun, Structural chemistry of uranium phosphonates, Coord. Chem. Rev. 303(2015) 86-109. |
| [15] | W.T. Yang, F.Y. Yi, T. Tian, W.G. Tian, Z.M. Sun, Structural variation within heterometallic uranyl hybrids based on flexible alkyldiphosphonate ligands, Cryst. Growth Des. 14(2014) 1366-1374. |
| [16] | A.T. Kerr, S.A. Kumalah, K.T. Holman, R.J. Butcher, C.L. Cahill, Uranyl coordination polymers incorporating η5-cyclopentadienyliron-functionalized η6-phthalate metalloligands:Syntheses, structures and photophysical properties, J. (Ⅰ)norg. Organomet. Polym. 24(2014) 128-136. |
| [17] | W.T. Yang, S. Dang, H. Wang, et al., Synthesis, structures, and properties of uranyl hybrids constructed by a variety of mono-and polycarboxylic acids, (Ⅰ)norg. Chem. 52(2013) 12394-12402. |
| [18] | H.Y. Wu, R.X. Wang, W.T. Yang, et al., 3-Fold-interpenetrated uranium-organic frameworks:new strategy for rationally constructing three-dimensional uranyl organic materials, (Ⅰ)norg. Chem. 51(2012) 3103-3107. |
| [19] | W.T. Yang, W.G. Tian, X.X. Liu, L. Wang, Z.M. Sun, Syntheses, structures, luminescence, and photocatalytic properties of a series of uranyl coordination polymers, Cryst. Growth Des. 14(2014) 5904-5911. |
| [20] | Siemens Analytical X-ray (Ⅰ)nstruments, (Ⅰ)nc., SMART and SA(Ⅰ)NT (software packages), Siemens Analytical X-ray (Ⅰ)nstruments, (Ⅰ)nc., Madison, W(Ⅰ), 1996. |
| [21] | Siemens (Ⅰ)ndustrial Automation, (Ⅰ)nc., SHELXTL Program, version 5.1, Siemens (Ⅰ)ndustrial Automation, (Ⅰ)nc., Madison, W(Ⅰ), 1997. |
| [22] | P.C. Burns, R.C. Ewing, F.C. Hawthorne, The crystal chemistry of hexavalent uranium:polyhedron geometries, bond-valence parameters, and polymerization of polyhedra, Can. Min. 35(1997) 1551-1570. |
| [23] | N.E. Brese, M. O'Keeffe, Bond-valence parameters for solids, Acta Cryst. B 47(1991) 192-197. |
| [24] | A. Mer, S. Obbade, M. Rivenet, C. Renard, F. Abraham,[La(UO2)V2O7][(UO2)(VO4)] the first lanthanum uranyl-vanadate with structure built from two types of sheets based upon the uranophane anion-topology, J. Solid State Chem. 185(2012) 180-186. |
| [25] | G. Meinrath, Uranium(V(Ⅰ)) speciation by spectroscopy, J. Radioanal. Nucl. Chem. 224(1997) 119-126. |
| [26] | P.O. Adelani, T.E. Albrecht-Schmitt, Syntheses of uranyl diphosphonate compounds using encapsulated cations as structure directing agents, Cryst. Growth Des. 11(2011) 4227-4237. |
 2016, Vol.27
2016, Vol.27