Diamide insecticides are newly-developed agrochemicals for pest control which are effective on lepidopterous insects [1]. Compared to the naturally sourced alkaloid ryanodine from Ryania speciosa [2, 3],artificial insecticides are less toxic to fish and mammals,because they selectively induce the release of Ca2+ through the insect ryanodine receptors (RyRs) [4, 5],but have no effect on mammalian RyRs [4, 6]. The selectivity of diamide insecticides may be the result of large species differences on the RyRs binding sites [7]. With a new mode of action,the diamide insecticides were favored for their safety to mammals,high efficiency,and lack of cross-resistance to pyrethroids,benzoylphenylureas,organophosphates,and carbamates [8, 9].
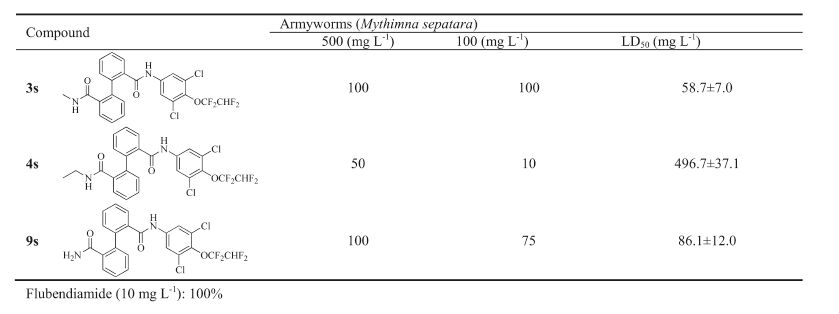
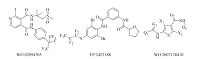
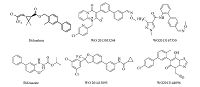
Basically,there are two kinds of diamide insecticides classified by chemical structures. One kind is the phthalic diamides,represented by flubendiamide,discovered by Nihon Nohyaku and jointly developed with Bayer CropScience [9]. The other kind is the anthranilamides,including chlorantraniliprole and cyantraniliprole developed by Dupont [10] (Fig. 1). Based on the general structure of phthalic diamide (Fig. 2),chemical modification of part B and C gave several novel candidates as potential Ryanodine insecticides [11, 12, 13, 14]. From the variation of part A,benzamides with a 3-formamide substituent have been reported recently by Syngenta [15, 16],Bayer [17, 18],Mitsui chemicals [19, 20] etc. (Fig. 3). In these examples,the two amides groups were not situated at adjacent positions as in the phthalic diamides or anthranilamides,but still showed high insecticidal activity. The biphenyl group occurs in pharmaceuticals and pesticides for its bioactivity. For example,the commercialized bifenthrin,bifenazate,and recent patents use biphenyl substitute as a pesticidal pharmacophore (Fig. 4) [21, 22, 23, 24]. Encouraged by the above description,we used the biphenyl group as the replacement of part A to discover more chemicals as insecticides. The two amides groups were located at the ortho-position of each aromatic ring. Herein,a series of biphenyl-diamides were synthesized and screened to investigate their insecticidal activity as a function of structure changes. Compound 1s containing 3,5-dichloro-4- (2,2,4,4-tetrafluoro-ethoxy)aniline shows growth inhibition to armyworms (Mythimna sepatara). It was found that Compound 3s substituted with formyl methylamine shows 100% mortality to armyworms at 100 mg L-1.

|
Download:
|
| Fig. 1.Commercialized diamide: flubendiamide,chlomathranilipole and cyantraniliprole. | |

|
Download:
|
| Fig. 2. General structure of phthatic amides and diphenic amides. | |
|
Download:
|
| Fig. 3. Insecticidal molecules with diamides subunit. | |
|
Download:
|
| Fig. 4.Biphenyl-containing pesticides. | |
All melting points were obtained with a Bu¨ chi Melting Point B540 and are uncorrected. NMR spectra were recorded in DMSO-d6 (1H at 400 MHz and 13C at 100 MHz) using TMS as the internal standard on a Bruker WP-400SY (400 MHz) spectrometer. Chemical shifts are reported in δ (parts per million) values. Highresolution mass spectra were recorded under electron impact (70 eV) condition using a MicroMass GCT CA 0= instrument. Analytical thin-layer chromatography (TLC) was carried out on precoated plates (silica gel 60 F254),and spots were visualized with ultraviolet (UV) light. Dichloromethane was treated with a 4A molecular sieve overnight. All other solvents and reagents were used as obtained from commercial sources without further purification.
General procedure for compounds 1-9: The diphenic anhydride was purchased from commercial source and recrystallized when necessary. According to Ref. [25],A solution of 2,4,6-trimethylpyridine (1.2 mmol) and aliphatic amine (1.2 mmol) in DMF (dimethyl formamide,1 mL) was added dropwise to the diphenic anhydride (1 mmol) in DMF (1 mL) at room temperature (Scheme 1). After 2 h of stirring,the solution was removed under reduced pressure. The residue was dissolved in 10 mL EtOAc (ethyl acetate),then washed with 15% HCl and brine. The product was evaporated and crystallized in EtOAc-petroleum ether.

|
Download:
|
| Scheme 1.Synthesis route of diphenic amides. | |
General procedure for the target compounds except 1c: Under argon condition [26],p-TsCl (p-toluenesulfonyl,1.2 mmol) in 2 mL dichloromethanewas injectedtothe2 mL dichloromethanemixture of compounds 1-9 (1 mmol) and 4-dimethylaminopyridine (2.4 mmol) at reflux temperature. The aromatic amine or heteroaromatic amine (1.2 mmol)was injected to the reactionmixture 2 h later. Themixturewas stirredforanother2 h. After removing solvent in vacuo,the residue was quenched with EtOAc and water,neutralized with sat. NaHCO3 solvent,the EtOAc layer was dried over anhydrous Na2SO4 and concentrated. The residue was chromatographed on silica gel (EtOAc-petroleum ether) to give target compounds. Thestructures of compounds 1a-9swere showed in the Supporting information.
Procedure for the preparation of compound 1c (N2-isopropyl-N2’- (4-nitrophenyl)-[1,1’-biphenyl]-2,20-dicarboxamide): Compound 1c was synthesized according to Ref. [27]. Diphenic anhydride (2 mmol) and 4-nitroaniline (2.4 mmol) were heated to reflux in chloroform for 3 h. After removing solvent in vacuo,the residue was resolved in EtOAc,then washed with NaHCO3 and brine. The intermediate was recrystallized in EtOAc-petroleum ether. The mixture of mono-acid intermediate 20-((4-nitrophenyl)carbamoyl)-[1,10-biphenyl]-2-carboxylic acid (1.0 mmol) and 4- dimethylaminopyridine (2.4 mmol) was heated (2.4 mmol) in dichloromethane to reflux,then injected with p-TsCl (1.2 mmol) and 2 mL dichloromethane for 2 h. Then,isopropylamine (1.2 mmol) was added to the reaction flask,followed by stirring for another 2 h. After removing the solvent in vacuo,the residue was quenched with EtOAc and water. After this was neutralized with sat. NaHCO3 solvent,the EtOAc layer was dried over anhydrous Na2SO4 and concentrated. The residue was chromatographed on silica gel (EtOAc-petroleum ether) to give target compound.
3. Results and discussion 3.1. SynthesisThe synthetic route of the designed structures was shown in Scheme 1. The characterization data for the target compounds can be found in the Supporting information. Refluxing 2,2- biphenyldicarboxylic acid in acetic anhydride gave the diphenic anhydride with a good yield. Using diphenic anhydride as staring material,the compounds 1-9 were synthesized with yields from =% to 90%,depending on the part of the aliphatic amines. The second amine was much more difficult to introduce into the core structure. The commonly-used amidation methods,such as acylation or condensation,usually lead to the formation of by-product 5H-dibenzo[c,e]azepine-5,7(6H)-dione. Thus,we used the p-toluenesulfonyl and compounds 1-9 to synthesize sulfonyl-O-acryl mixed anhydrides as an alternative routine. The mixed anhydrides reacted with amines to give the final products. However,in preparing compound 1c,treatment of mixed anhydride with the p-nitroaniline could not give the expected diamide. This might be caused by the low nucleophilicity of the p-nitroaniline. Compound 1c was prepared under different reaction conditions via amidation sequence. The pnitroaniline was introduced under refluxing chloroform with diphenic anhydride to form the mono-amide intermediate 20- ((4-nitrophenyl)carbamoyl)-[1,10-biphenyl]-2-carboxylic acid. Then,this mono-amide intermediate was treated with ptoluenesulfonyl,followed by i-propylamine to give compound 1c.
3.2. Insecticidal activityThe insecticidal activities of the target compounds were evaluated against armyworms (M. sepatara). Compounds 3s,4s and 9s containing 3,5-dichloro-4-(2,2,4,4-tetrafluoro-ethoxy)aniline group were insecticidal with mortality from 75% to 100% at 500 mg L-1 (Table 1). When R1 was i-propyl,the compound 1s showed growth inhibition to armyworms at 500 mg L-1,while compound 8s with a larger aliphatic group is inactive. These differences suggested that the smaller size of substituent R1 was favorable to bioactivity. When R2 was some other aromatic amine or heterocyclic ring,the compounds were inactive. This indicated that the insecticidal activities of these compounds do not depend on the electrical properties of the substituent. Compound 3s with the shortest aliphatic chain showed LD50 value of 58.7 mg L-1 to armyworm. Compound 3s showed the best water solubility of compounds 3s,4s,and 9s. A tentative inference was that the different insecticidal activities of compound 3s,4s,and 9s might be related to their physiochemical property which could affect drug absorption and delivery.
|
|
Table 1 Insecticidal activity of target compounds to armyworms. |
The symptoms of larvae poisoned by 3s were investigated. In contrast to the commercial diamide insecticides,it takes effect in a longer time. Larvae poisoned by 3s first showed growth inhibition with smaller body size than the controlled group. Then,the armyworms died of dehydration,and the body shriveled. The dead worms left a small hard residue,which is similar to the symptoms caused by flubendiamide.
4. ConclusionA series of novel diphenic diamides were designed and synthesized. The insecticidal activities were assayed to give a potential candidate 3s. It was suggested that the separation of diamides to a diphenic scaffold could be feasible,while the most suitable substituents could be changed. The insecticidal compound 3s could be used as a lead compound for further research. Appendix A. Supplementary data Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.12.011.
| [1] | G.P. Lahm, D. Cordova, J.D. Barry, New and selective ryanodine receptor activators for insect control, Bioorg. Med. Chem. 17(2009) 4127-4133. |
| [2] | E.F. Rogers, F.R. Koniuszy, J. Shaverl Jr., K. Folkers, Plant insecticides. (Ⅰ). Ryanodine, a new alkaloid from Ryania speciosa vahl, J. Am. Chem. Soc. 70(1948) 3086-3088. |
| [3] | P.R. Jefferies, R.F. Toia, J.E. Casida, Ryanodyl 3-(pyridine-3-carboxylate):a novel ryanoid from ryania insecticide, J. Nat. Prod. 54(1991) 1147-1149. |
| [4] | U. Ebbinghaus-Kintscher, P. Luemmen, N. Lobitz, et al., Phthalic acid diamides activate ryanodine-sensitive Ca2+ release channels in insects, Cell Calcium 39(2006) 21-33. |
| [5] | D. Cordova, E.A. Benner, M.D. Sacher, et al., Anthranilic diamides:a new class of insecticides with a novel mode of action, ryanodine receptor activation, Pest. Biochem. Physiol. 84(2006) 196-214. |
| [6] | D.B. Sattelle, D. Cordova, T.R. Cheek, (Ⅰ)nsect ryanodine receptors:molecular targets for novel pest control chemicals, (Ⅰ)nvert. Neurosci. 8(2008) 107-119. |
| [7] | S.Z. Qi, J.E. Casida, Species differences in chlorantraniliprole and flubendiamide insecticide binding sites in the ryanodine receptor, Pest. Biochem. Physiol. 107(2013) 321-326. |
| [8] | R. Nauen, (Ⅰ)nsecticide mode of action:return of the ryanodine receptor, Pest. Manage. Sci. 62(2006) 690-692. |
| [9] | M. Tohnishi, H. Nakao, T. Furuya, et al., Flubendiamide, a novel insecticide highly active against lepidopterous insect pests, J. Pestic. Sci. 30(2005) 354-360. |
| [10] | G.P. Lahm, T.M. Stevenson, T.P. Selby, et al., RynaxypyrTM:a new insecticidal anthranilic diamide that acts as a potent and selective ryanodine receptor activator, Bioorg. Med. Chem. Lett. 17(2007) 6274-6279. |
| [11] | C. Gnamm, A. Jeanguenat, A.C. Dutton, et al., Novel diamide insecticides:sulfoximines, sulfonimidamides and other new sulfonimidoyl derivatives, Bioorg. Med. Chem. Lett. 22(2012) 3800-3806. |
| [12] | T. Yan, S.J. Yu, P.F. Liu, et al., Design, Synthesis and biological activities of novel benzoyl hydrazines containing pyrazole, Chin. J. Chem. 30(2012) 919-923. |
| [13] | M.L. Feng, Y.F. Li, H.J. Zhu, et al., Synthesis, insecticidal activity, and structureactivity relationship of trifluoromethyl-containing phthalic acid diamide structures, J. Agric. Food Chem. 58(2010) 10999-11006. |
| [14] | M. Liu, Y. Wang, W.Z. Wangyang, et al., Design, synthesis, and insecticidal activities of phthalamides containing a hydrazone substructure, J. Agric. Food Chem. 58(2010) 6858-6863. |
| [15] | P.J.M. Jung, P. Durieux, W. Lutz, et al., (Ⅰ)nsecticidal compounds, WO 2007128410(2007). |
| [16] | O.F. Hueter, P.J.M. Jung, T. Pitterna, H. Smits, A. Stoller, (Ⅰ)nsecticidal compounds, WO 2014067838(2014). |
| [17] | A. Yanagi, Y. Watanabe, K. Wada, et al., (Ⅰ)nsecticidal 3-acylaminobenzanilides, WO 2007017075(2007). |
| [18] | A. Yanagi, Y. Watanabe, J. Mihara, et al., (Ⅰ)nsecticidal 2-acylaminothiazole-4-carboxamides, WO 2007051560(2007). |
| [19] | K. Yoshida, T. Wakita, H. Katsuta, A. Kai, et al., Agricultural/horticultural insecticide and method for using the same, EP 2324188(2003). |
| [20] | K. Yoshida, Y. Kobayashi, M. Nomura, et al., Amide derivative, pesticide containing such compound and use thereof, WO 2006137395(2006). |
| [21] | L. Lu, J.Y. Cassayre, T. Luksch, et al., Dihydrobenzofuran derivatives as insecticidal compounds, WO 2014135095(2014). |
| [22] | C. Dubost, P.Y. Coqueron, 5-Halogenopyrazole biphenylcarboxamides, WO 2013167550(2013). |
| [23] | A. Narine, J. Dickhaut, F. Kaiser, et al., Substituted mesoionic imine compounds for combating animal pests, WO 2014033244(2014). |
| [24] | C. Waldraff, S. Lehr, A. Angermann, et al., Herbicidally and insecticidally active thiazolopyridinones, WO 2013144096(2013). |
| [25] | (Ⅰ).L. Karle, P. Venkateshwarlu, R. Nagaraj, et al., Diphenic acid as a general conformational lock in the design of bihelical structures, Chem. Eur. J. 13(2007) 4253-4263. |
| [26] | R.P. Robinson, J.A. Bartlett, P. Bertinato, et al., Discovery of microsomal triglyceride transfer protein (MTP) inhibitors with potential for decreased active metabolite load compared to dirlotapide, Bioorg. Med. Chem. Lett. 21(2011) 4150-4154. |
| [27] | C.J. Perry, Z. Parveen, The cyclisation of substituted phthalanilic acids in acetic acid solution:a kinetic study of substituted N-phenylphthalimide formation, J. Chem. Soc. Perkin Trans. 2(2001) 512-521. |
 2016, Vol.27
2016, Vol.27