b. Department of Chemistry, Nankai University, Tianjin 300071, China
Since the observation of slow magnetic relaxation in a cobalt(Ⅱ) radical alternating chain opened the door to single chain magnets (SCMs) [1],this interesting field has gained considerable attention from chemists and physicists and has been rapidly expanding,owing to their potential applications in high-density information storage,quantum computation,and molecular spintronic [2, 3, 4, 5]. Inspiring progress have been made in the field of SCMs with the development of crystal engineering and molecule-based materials,and some predesigned or existing properties and functions could be achieved or mediated at the molecular level [6, 7]. To construct SCMs,two basic respects need to be considered: strong uniaxial Ising-type anisotropy,strong intrachain and weak interchain magnetic interaction. According to the requirements,Gao et al. [8] summarized three effective strategies to the fabrication of SCMs,ferromagnetic (FO),ferrimagnetic (FI),and weak ferromagnetic (WF) approaches. However,the rational design of such molecular materials is still a challenging task for researchers due to the difficulties in both structural and magnetic controls.
Hitherto,current research mainly utilizes two effective approaches to build SCMs. The most common approach is serendipitous assembly between metal ions with large singleion anisotropy such as Mn(Ⅲ),Co(Ⅱ),Fe(Ⅱ) and Ln(Ⅲ) ions [8, 9, 10, 11] and suitable ligands that could efficiently mediate intrachain exchange interactions so as to get simultaneously isolated chains to avoid interchain exchange interactions [12, 13, 14]. Although it is not easy to foresee the concrete bonding modes of the resultant products,the approach have been successfully utilized in many related systems. Parallel to the serendipitous assembly,step-bystep assembly has also been well used to obtain the desired products,especially the assembly of pre-existing magnetic units offering the opportunities of targeted synthesis. The feasibility of such two approaches to create SCMs has been well verified in literatures [15].
We have sought to explore the synthetic methodology toward fabricating new SCMs. For example,SCM-like behavior was observed in a unique substituted 3D Co(Ⅱ)-formate framework with serendipitous assembly approach [2]. Because of the isolation of isonicotine and the strong anisotropy of spin-canting,the 1D WF chains exhibit slow magnetic relaxations,adding a new member of SCM based on the WF chains. A 2D coordination polymer displaying SCM-like behavior has also been constructed via serendipitous assembly approach [16]. Because the long interchain spacer 1,4-bis(imidazol-1-yl) benzene prevents the magnetic ordering,the FO cobalt chains bridged by μ2-1,1-azido display slow relaxation of magnetization,providing a new case of SCM based on the F chains. As our continuous studies on the synthesis and magnetic investigation of SCMs,in this work we report that the step-by-step assembly of [Cr3O(Bzo-t-Bu)6(H2O)3]Bzo-t-Bu (HBzot-Bu = 4-tert-butylbenzoic acid; Cr3O-Bzo-t-Bu,for short) and Dy(NO3)3-6H2O with 1,1,1-tris(hydroxymethyl)ethane (H3thmp) gives rise to a fascinating heterometallic one-dimensional (1D) coordination framework [Cr2Dy3(Bzo-t-Bu)9(thmp)2]-4H2O (1) which features slow relaxation of the magnetization (vide infra). To reach a balance between the structural and magnetic controls,a certain degree of "design" is indeed present in our selection of the starting materials: (i) Cr(Ⅲ) with moderate spin ground state (S) [17] and Dy(Ⅲ) ions with strong Ising-like magnetic anisotropy [18, 19] were selected to favor the creation of an energy barrier for the magnetization reversal in SCMs. (ii) The carboxylate [20] and polyalcohol ligands [21] exhibit abundant versatility and diversity in bridging metal ions and magnetic transmitting,which have been well used to construct single-molecule magnets (SMMs) and SCMs. (iii) The Bzo-t-Bu ligand with steric effect serves as separator to prevent the extension of coordination framework. With these considerations in mind,we successfully isolated the blue block crystals of 1 and alternating-current (AC) signals indicate the presence of a frequency-dependent slow magnetic relaxation for complex 1.
2. Experimental[Cr2Dy3(Bzo-t-Bu)9(thmp)2]-4H2O (1) was synthesized by the solvothermal reaction of Cr3O-Bzo-t-Bu,Dy(NO3)3-6H2O and H3thmp in the presence of triethylamine (TEA) in mixed CH3CN-H2O solvent at 140 ℃. Detailed experimental method and crystallographic data are summarized in Supporting information.
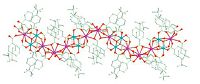
3. Results and discussion 3.1. Description of crystal structureComplex 1 crystallizes in a triclinic system with space group P-1 and Z = 2. There are two CrⅢ ions,three DyⅢ ions,nine Bzo-t-Bu-ligands and two thmp3- anions in the asymmetric unit of 1,whose molecular structure is presented in Fig. 1. All H3thmp ligands exhibit the µ4-η3:η2:η2-bridging mode and exist in the fully deprotonated form thmp3- (Fig. 1a). The coordination mode of Bzo-t-Bu-groups falls into three categories: the first coordinates to one DyⅢ and one CrⅢ ion in syn-syn-µ2-η1:η1-bridging mode (Fig. 1b); the second connects two DyⅢ ions in µ2-η1:η2-bridging mode (Fig. 1c); the third links three DyⅢ ions in µ3-η2:η2-bridging mode (Fig. 1d). All Cr or Dy sites are oxygen-coordinated. The octahedral geometry of the Cr(1)/Cr(2) ion is [CrO6],in which two Ocarboxylate atoms are from two Bzo-t-Bu-groups and four Ohydroxyl atoms from two thmp3- ligands. Dy(1) ion is coordinated with eight O atoms belonging to five Bzo-t-Bu-ligands and three Ohydroxyl atoms from two thmp3- groups,showing a square antiprism geometry. The Dy(2) center is ten coordinate,and the [O10] coordination atoms are from five η1:η1-chelating Bzo-t-Bu-ligands. The Dy(3) ion displays a square antiprism geometry formed by the coordination of five Ocarboxylate atoms from five Bzot-Bu-ligands and three Ohydroxyl atoms from two thmp3- groups. Two thmp3-groups and four Bzo-t-Bu-ligands bridge two CrⅢ ions and two DyⅢ ions to form heterometallic tetrameric [Cr2Dy2(thmp)2(Bzo-t-Bu)4]2+ units,which are further bridged by [Dy(Bzo-t-Bu)5]2-units to form multinuclear 1D chain structure (Fig. 2). The Cr-O bond distances range from 1.948(7)Å to 2.036(7)A˚ ,while the Dy-O bond distances are from 2.313(8) Å to 2.588(7)A˚ . Notably,for the steric hindrance of ligand Bzo-t-Bu-,the introduction of Bzo-t-Bu-may be favorable to form lowdimensional structure,and the thmp3- ligand with more coordination sites is to the benefit of multinuclear structure.

|
Download:
|
| Fig. 1. Binding modes of H3thmp and Bzo-t-Bu-ligands (turquoise,Cr; pink,Dy; red,O; light green,C). | |
|
Download:
|
| Fig. 2.1D chain structure of the complex 1 | |
To confirm the phase purities of complex 1 before magnetic measurements,PXRD analyses was performed. As shown in Fig. S1 in Supporting information,the good consistence between the observed PXRD and the predicted one indicated the phase purities of the sample.
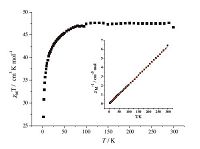
3.3. Magnetic studiesThe variable-temperature magnetic susceptibility study of complex 1 was carried out at an applied field of 1000 Oe in the temperature range of 2-300 K,as shown in Fig. 3. The observed xMT is 47.6 cm3 K mol-1 at 300 K for 1,which is in agreement with the theoretical value of 46.23 cm3 K mol-1 for the unit of three isolated DyⅢ (42.48 cm3 K mol-1,6H15/2,g = 3/4) ions and two noninteracting CrⅢ ions (3.75 cm3 K mol-1,s = 3/2,g = 2). Upon cooling,the xMT value of 1 stays essentially constant until 100 K,then followed by an obvious decrease to the minimum value of 26.96 cm3 K mol-1 at 2 K,which is mainly ascribed to the overall antiferromagnetic interactions and/or the thermal depopulation of the excited Stark sublevels. The Stark sublevels of DyⅢ of the anisotropic DyⅢ ions may be progressively thermally depopulated,leading to a decrease of the xMT value [22]. The temperature dependence of the reciprocal susceptibility (1/xM) obeys the Curie-Weiss law at 2-300 K with C = 47.9 cm3 K mol-1 and u = -2.3 K. The negative u value cannot indicate antiferromagnetic interactions,because the crystal field effect of DyⅢ or the spin-orbit coupling of heavy rare earth ions are also result in the negative θ value.
|
Download:
|
| Fig. 3.Temperature dependence of the xMT and xM -1 curve for 1. The red lines represent the best fit to the Curie-Weiss law. | |
The M vs. H data measured in different magnetic fields at 2 K show a steady increase at low field (<1 T),and then M reaches a value of 21.1 Nβ at 2 K and 7 T,far from the theoretical saturated value of 36 Nβ for three DyⅢ (g = 4/3,J = 15/2) and two CrⅢ (g = 2,S = 3/2) (Fig. 4). In the high-field region,the increase of magnetization is slow and linear,which may be attributed to the anisotropy of the sample. The magnetic unsaturation even at 7 T may be attributed to the magnetic anisotropy and/or low-lying excited states of DyⅢ ions [23].

|
Download:
|
| Fig. 4.M vs. H plot at 2 K for 1 | |
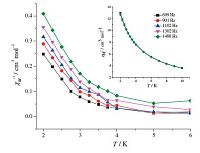
To further explore the magnetic dynamics of 1,the frequency and temperature dependencies of the alternating current (AC)susceptibilities were collected under a zero direct current (DC) field and a 3 Oe AC magnetic field. As shown in Fig. 5,frequencydependent out-of-phase signals for complex 1 are observed below 4 K,yet all of the in-phase curves (x') are almost consistent without peaks,suggesting the slow magnetic relaxation behavior [24],which might be an indication of SCM behavior. However,no peak maximum is found in the technically available temperature range. This may result from that quantum tunneling of the magnetization (QTM) is too fast at the operating limits of our magnetometer. Because slow relaxation of the magnetization is experimentally observed only over a short range of temperature and the absence of maxima in the x'' vs. T,Arrhenius equation cannot be fit to evaluate the energy barrier (DE/kB) and the preexponential factor (τ0).
|
Download:
|
| Fig. 5.Temperature dependence of the out-of-phase signals of the ac susceptibility for 1 under a zero dc field (Hac = 3 Oe). | |
In conclusion,with [Cr3O(Bzo-t-Bu)6(H2O)3]Bzo-t-Bu precursor as CrⅢ ion sources,a new [Cr2Dy3] cluster-based heterometallic one-dimensional (1D) complex has been prepared via stepwise strategy. Magnetic measurements indicate weak antiferromagnetic interactions and/or the thermal depopulation of the excited Stark sublevels in the complex 1. And AC measurements indicate that complex 1 exhibits slow magnetic relaxation.
AcknowledgmentsThis work was supported by the NSF of China (Nos. 21031002,21421001),and Natural Science Fund of Tianjin,China (No. 15JCZDJC38800).
Appendix A. Supplementary dataCrystallographic data file (CIF),Section 2,simulated and experimental PXRD (Fig. S1) of 1,crystal data and structure refinement parameters and selected bond distances and angles for 1 (Tables S1-S3). Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2016.01.001.
| [1] | R.J. Glauber, Time-dependent statistics of the ising model, J. Math. Phys. 4(1963) 294-307. |
| [2] | A.V. Palii, O.S. Reu, S.M. Ostrovsky, et al., A highly anisotropic cobalt(Ⅱ)-based single-chain magnet:exploration of spin canting in an antiferromagnetic array, J. Am. Chem. Soc. 130(2008) 14729-14738. |
| [3] | Y.Z. Zheng, W. Xue, M.L. Tong, X.M. Chen, S.L. Zheng, Probing single-chain magnets in a family of linear chain compounds constructed by magnetically anisotropic metal-ions and cyclohexane-1,2-dicarboxylate analogues, (Ⅰ)norg. Chem. 47(2008) 11202-11211. |
| [4] | T.F. Liu, D. Fu, S. Gao, et al., An azide-bridged homospin single-chain magnet:[Co(2,20-bithiazoline)(N3)2]n, J. Am. Chem. Soc. 125(2003) 13976-13977. |
| [5] | E. Coronado, J.R. Galan-Mascarós, C. Martí-Gastaldo, Single chain magnets based on the oxalate ligand, J. Am. Chem. Soc. 130(2008) 14987-14989. |
| [6] | D. Maspoch, D. Ruiz-Molina, K. Wurst, et al., A nanoporous molecular magnet with reversible solvent-induced mechanical and magnetic properties, Nat. Mater. 2(2003) 190-195. |
| [7] | J.Z. Lu, D.R. Turner, L.P. Harding, et al., Octapi interactions:self-assembly of a Pdbased[2] catenane driven by eightfold π interactions, J. Am. Chem. Soc. 131(2009) 10372-10373. |
| [8] | H.L. Sun, Z.M. Wang, S. Gao, Strategies towards single-chain magnets, Coord. Chem. Rev. 254(2010) 1081-1100. |
| [9] | J.Y. Zhang, K. Wang, X.B. Li, E.Q. Gao, Magnetic coupling and slow relaxation of magnetization in chain-based MnⅡ, CoⅡ, and NiⅡ coordination frameworks, (Ⅰ)norg. Chem. 53(2014) 9306-9314. |
| [10] | Y.Q. Zhang, C.L. Luo, X.B. Wu, B.W. Wang, S. Gao, Magneto-structural correlations in a family of FeⅡRe(Ⅰ)V(CN)2 single-chain magnets:density functional theory and Ab initio calculations, (Ⅰ)norg. Chem. 53(2014) 3503-3510. |
| [11] | E.V. Peresypkina, A.M. Majcher, M. Rams, K.E. Vostrikova, A single chain magnet involving hexacyanoosmate, Chem. Commun. 50(2014) 7150-7153. |
| [12] | R. Lescouëzec, J. Vaissermann, C. Ruiz-Pé rez, et al., Cyanide-bridged iron(Ⅲ)-cobalt(Ⅱ) double Zigzag ferromagnetic chains:two new molecular magnetic nanowires, Angew. Chem. (Ⅰ)nt. Ed. Engl. 42(2003) 1483-1486. |
| [13] | X.T. Liu, X.Y. Wang, W.X. Zhang, P. Cui, S. Gao, Weak Ferromagnetism and dynamic magnetic behavior in a single end-to-end azide-bridged nickel(Ⅱ) chain, Adv. Mater. 18(2006) 2852-2856. |
| [14] | X.J. Li, X.Y. Wang, S. Gao, R. Cao, Two three-dimensional metal-organic frameworks containing one-dimensional hydroxyl/carboxylate mixed bridged metal chains:syntheses, crystal structures, and magnetic properties, (Ⅰ)norg. Chem. 45(2006) 1508-1516. |
| [15] | J. Tao, H. Maruyama, O. Sato, Valence tautomeric transitions with thermal hysteresis around room temperature and photoinduced effects observed in a cobalt-tetraoxolene complex, J. Am. Chem. Soc. 128(2006) 1790-1791. |
| [16] | Y.Z. Zheng, M.L. Tong, W.X. Zhang, X.M. Chen, Assembling magnetic nanowires into networks:a layered CoⅡ carboxylate coordination polymer exhibiting singlechain-magnet behavior, Angew. Chem. (Ⅰ)nt. Ed. Engl. 45(2006) 6310-6314. |
| [17] | C.R. Staples, (Ⅰ).K. Dhawan, M.G. Finnegan, et al., Electronic, magnetic, and redox properties of[MFe3S4] clusters (M=Cd, Cu, Cr) in pyrococcus furiosus ferredoxin, (Ⅰ)norg. Chem. 36(1997) 5740-5749. |
| [18] | K. Katoh, R. Asano, A. Miura, et al., Effect of f-f interactions on quantum tunnelling of the magnetization:mono-and dinuclear Dy(Ⅲ) phthalocyaninato triple-decker single-molecule magnets with the same octacoordination environment, Dalton Trans. 43(2014) 7716-7725. |
| [19] | Z.L. Wu, J. Dong, W.Y. Ni, et al., pH-induced Dy4 and Dy10 cluster-based 1D chains with different magnetic relaxation features, Dalton Trans. 43(2014) 16838-16845. |
| [20] | A.J. Tasiopoulos, A. Vinslava, W. Wernsdorfer, K.A. Abboud, G. Christou, Giant single-molecule magnets:a {Mn84} torus and its supramolecular nanotubes, Angew. Chem. (Ⅰ)nt. Ed. Engl. 43(2004) 2117-2121. |
| [21] | V. Chandrasekhar, P. Bag, M. Speldrich, J. van Leusen, P. Kö gerler, Synthesis, structure, and magnetic properties of a new family of tetra-nuclear {Mn2ⅢLn2}(Ln=Dy, Gd, Tb, Ho) clusters with an arch-type topology:singlemolecule magnetism behavior in the dysprosium and terbium analogues, (Ⅰ)norg. Chem. 52(2013) 5035-5044. |
| [22] | J.W. Sharples, Y.Z. Zheng, F. Tuna, E.J.L. Mc(Ⅰ)nnesa, D. Collison, Lanthanide discs chill well and relax slowly, Chem. Commun. 47(2011) 7650-7652. |
| [23] | S.D. Han, X.H. Miao, S.J. Liu, X.H. Bu, Magnetocaloric effect and slow magnetic relaxation in two dense (3,12)-connected lanthanide complexes, (Ⅰ)norg. Chem. Front. 1(2014) 549-552. |
| [24] | X.H. Miao, S.D. Han, S.J. Liu, X.H. Bu, Two lanthanide(Ⅲ)-copper(Ⅱ) chains based on[Cu2Ln2] clusters exhibiting high stability, magnetocaloric effect and slow magnetic relaxation, Chin. Chem. Lett. 25(2014) 829-834. |
 2016, Vol.27
2016, Vol.27 


