b. Department of Chemistry, University of Science and Technology of China, Hefei 230026, China
Chiral a-amino acids are the essential building blocks of bioactive peptides and proteins. They have found broad applications in the fields of drug development and protein engineering [1].The incorporation of unnatural a-amino acids renders synthetically modified natural biologics with novel structures and interesting properties [2]. For example,compared with the natural peptides and proteins,their synthetic derivatives containing unnatural a-amino acids may exhibit enhanced bioactivities,selectivity,and improved enzymatic stabilities. In addition to the use as the subunits of bioactive molecules,unnatural a-amino acids have also been widely used in modern synthetic organic chemistry and even total synthesis either as chiral ligands or substrates [3].
The short of operationally-easy and cost-effective methods for the preparation of unnatural amino acids is an often-encountered problem in many research programs [4]. Although strategies such as asymmetric synthesis [5] or chiral resolution [6] have been available,it remains an important challenge to develop more convenient and less expensive approaches to synthesize chiral amino acids. Ideally,it would be of great practical interest if we can directly modify the readily available natural amino acids and peptides to make their derivatives.
In recent years,C-H functionalization reactions have emerged as powerful methodologies for the synthesis of various organic molecules [7]. From the perspective of atom-economy and stepefficiency,C-H functionalization should also provide an efficient strategy for the synthesis of unnatural amino acids [8]. In particular,thanks to the great advance for the functionalization of un-activated C(sp3)-H bonds,increasing attentions have been directed to the modification of C(sp3)-H bonds of the side chains of natural amino acids and even peptides. In this article,we review the recent progress in Pd-catalyzed functionalization of β-C(sp3)- H and g-C(sp3)-H bonds to modify the side chains of amino acids and peptides.
2. C(sp3)-H activation/C-O couplingIn 2006,Corey et al. reported one of the first examples for the C(sp3)-H acetoxylation process of the N-phthaloyl a-amino amides [9]. In this pioneering work,8-aminoquinoline (Q) was found to be the only suitable directing group for this particular C-H functionalization reaction. The derivatives of several natural amino acids,for instance,alanine (1),leucine (2) and phenylalanine (3) were converted to the corresponding β-acetoxylation products smoothly with modest to good yields and high diastereoselectivity.
The optimal catalyst system was composed of Pd(OAc)2,oxone,Ac2O,Mn(OAc)2 and CH3NO2 (Scheme 1). The authors proposed that this reaction might proceed through a Pd(Ⅱ)-mediated C(sp3)- H insertion mechanism that had been established in the previous studies [10] (Fig. 1). Pd(Ⅱ) coordinated to the substrate to form intermediate 4. Then a doubly five-membered palladacycle 5 was produced through C-H insertion. Intermediate 5 was subsequently oxidized to a Pd(Ⅳ) species 6. Finally,C-O reductive elimination took place from 6 to generate the product and finish the catalytic cycle. For the observed stereoselectivity at the β-position,it could be attributed to the formation of a sterically favored transpalladacycle 5,in which the R group and N-Phth group prefer to adopt a trans-orientation. In a related work,Chen et al. reported the Pd-catalyzed N-picolinoyl (PA)-directed g-C(sp3)-H alkoxylation reaction in 2012 [11].
|
Download:
|
| Scheme 1.Pd-catalyzed 8-aminoquinoline directed C(sp3)-H acetoxylation.Phth = phthaloyl. | |

|
Download:
|
| Fig. 1.Proposed mechanism for Pd-catalyzed 8-aminoquinoline directed C(sp3)-H acetoxylation. | |
Recently,Shi research group reported an improved conditions for the C-O coupling reaction at the β-C(sp3)-H bond of protected amino acids [12]. In this work,the authors demonstrated the removal of directing and protecting groups (Scheme 2). This transformation provides an effective and convenient way for the preparation of β-mercapto-a-amino acids,which are important substrates for the native chemical ligation (NCL) needed for protein chemical synthesis [13]. Furthermore,Yu accomplished the siteselective C(sp3)-H acetoxylation in tripeptides [14]. It should be pointed out that the N-terminus is limited to alanine at present.

|
Download:
|
| Scheme 2.Removal of the Phth protecting group and 8-aminoquinoline directing group. DCE = 1,2-Dichloroethane. | |
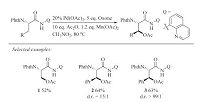
In 2012,Chen et al. developed a Pd-catalyzed intramolecular amination reaction at the C(sp3)-H bonds of the methyl groups in PA-protected amide substrates [15]. This reaction provides an effective method for the synthesis of azetidine and pyrrolidine derivatives (especially a-amino acids containing four or fivemembered rings). Pd(OAc)2/PhI(OAc)2/AcOH was found to constitute an efficient catalytic system for this reaction (Scheme 3).

|
Download:
|
| Scheme 3.Pd-catalyzed PA-directed intramolecular amination with C(sp3)-H bonds. | |
Soon later,Chen and co-workers described a Pd-catalyzed carboxamide-directed intramolecular amination reaction of the methyl group of the amino acid substrates [16]. 8-Aminoquinoline (Q) and 2-picolylamine (PM) were found to be suitable directing groups for this transformation (Scheme 4). The proposed structures of the Pd(Ⅱ) intermediates in the Pd-catalyzed intramolecular amination reaction with C(sp3)-H bonds were shown in Fig. 2. A Pd(Ⅱ)/Pd(Ⅳ) mechanism was proposed by the authors.
|
Download:
|
| Scheme 4.Pd-catalyzed intramolecular amination with Q or PM directing groups. | |

|
Download:
|
| Fig. 2.Proposed structures of the Pd(Ⅱ) intermediates in the Pd-catalyzed intramolecular amination with C(sp3)-H bonds. | |
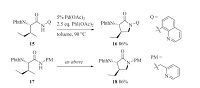
Meanwhile,Shi et al. reported a Pd-catalyzed 2-(pyridin-2-yl)- isopropylamine (PIP) amide directed sequential C(sp3)-H arylation/ amidation reaction [17]. As a newly developed directing group,PIP was found to render a better selectivity for the monoarylation process with enhanced reactivity (Scheme 5).
|
Download:
|
| Scheme 5.Pd-catalyzed PIP-directed intramolecular sequential C(sp3)-H arylation/ amidation reaction. DMPU = 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone. | |
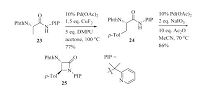
In 2014,Shi et al. described a Pd-catalyzed mono-g-borylation reaction of PA-containing amino acids [18]. To prevent the formation of palladium black,iPr2S was added as the additive.
Furthermore,O2 was chosen as the oxidant whereas the other oxidants failed to provide satisfactory results (Scheme 6). Under the optimal reaction conditions,valine (26),isoleucine (27),O-tertbutyl threonine (28) and tert-leucine (29) derivatives were successfully mono-g-borylated with good yields. The authors proposed that this reaction might proceed a Pd(Ⅱ)/Pd(0) pathway where Pd(Ⅱ) species was produced for C-H activation. The desired products and Pd(0) were generated through reductive elimination.Pd(O) was re-oxidized in the presence of O2 to finish the catalytic cycle.
|
Download:
|
| Scheme 6.Pd-catalyzed PA-directed C(sp3)-H borylation. Pin = OC(Me2)C(Me2)O. | |
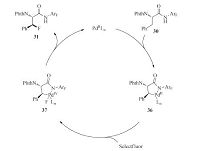
In 2015,Yu and coworkers described a Pd-catalyzed β-C(sp3)-H stereoselective fluorination reaction of a-amino acids [19].Selectfluor was used as the electrophilic fluorination reagents.The quinoline-based ligand L1 was demonstrated to be crucial for the occurrence of this reaction. In the presence of Pd(TFA)2,quinoline-based ligands,Ag2CO3 and Selectfluor in 1,4-dioxane,a wide range of β-fluoro-a-amino acids were successfully produced.
The authors suggested that this reaction might involve a Pd(Ⅱ)/ Pd(Ⅳ) catalytic cycle (Fig. 3). The initiation step was the formation of palladacycle 36 through C-H activation. Then,intermediate 37 was formed through effect of Selectfluor. Finally,the desired product was obtained through the C-F reductive elimination on Pd(Ⅳ) species. In intermediate 37,Ph group and N-Phth group adopted a trans-orientation and the configuration at β-position was retained during the reductive elimination step. Later,Shi [20] and Ge et al. [21] independently reported Pd-catalyzed fluorination reactions of the un-activated methylene C(sp3)-H bonds of amino acids. PIP was used as the directing group (Scheme 7).
|
Download:
|
| Fig. 3.Proposed mechanism for Yu’s ligand-enabled Pd-catalyzed C(sp3)-H fluorination. | |

|
Download:
|
| Scheme 7.Pd-catalyzed C(sp3)-H fluorination of a-amino acids.TFA = trifluoroacetic acid. Seclectfluor = 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate). | |
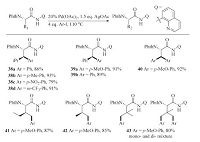
In 2006,the Corey’s group reported the Pd-catalyzed C(sp3)-H arylation reaction of N-phthaloyl amino acids [9]. 8-Aminoquinoline was used as the directing group. Note that the use of 8- aminoquinoline as the directing group was previously reported by Daugulis et al. [10c] Because leucine (38a-d) and phenylalanine (39a-b) derivatives contain secondary C(sp3)-H bonds,they were arylated at the β-position. On the other hand,alanine (40) derivatives were diarylated at the terminal C(sp3)-H. For isoleucine (41) and valine (42),mono-arylation occurred at the g-C(sp3)- H bonds. Finally,a tert-leucine (43) derivative was converted to a mixture of mono- and diarylated products (Scheme 8).
|
Download:
|
| Scheme 8.Pd-catalyzed 8-aminoquinoline directed C(sp3)-H arylation reaction of amino acids. | |
Daugulis et al. found that the use of 2-thiomethylaniline as directing group could lead to mono-arylated alanine derivatives [22]. In this work,Daugulis et al. demonstrated the removal of both 8-aminoquinoline and 2-thiomethylaniline. [22] Shi et al. reported the use of PIP as the directing group for the mono-arylation of alanine derivatives [16]. The PIP group was stable to the oxidative conditions,thus providing additional opportunities for further intramolecular amination.
The widely accepted mechanism for Pd-catalyzed directed C(sp3)-H arylation reaction involved a Pd(Ⅱ)/Pd(Ⅳ) pathway [23] (Fig. 4). However,the involvement of a Pd(Ⅲ) intermediate could not be completely excluded [24].

|
Download:
|
| Fig. 4.Proposed mechanism for Pd-catalyzed 8-aminoquinoline directed C(sp3)-H arylation reaction. | |
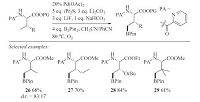
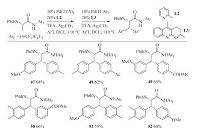
In 2014,Yu et al. described ligand-controlled arylation of the β- C(sp3)-H bonds [25]. It provides an additional example for the ligand-controlled C-H activation reactions. The reactivity and selectivity of transition metal-catalyzed C-H activation process were tuned by the use of different ligands. Pyridine derived ligand L2 promoted the mono-arylation of alanine derivatives and quinoline family ligand L3 led to the further di-arylation products.
This strategy enabled the sequential asymmetric di-arylation of the methyl group of alanine derivatives. By using this method,a broad range of β-Ar-β-Ar0-a-amino acids could be synthesized (Scheme 9). More recently,Yu and coworkers reported the Pdcatalyzed β-C(sp3)-H mono-arylation reaction with arylsilanes.
|
Download:
|
| Scheme 9.Pd-catalyzed ligand-controlled sequential C(sp3)-H asymmetric diarylation in one pot. | |
This reaction is also enabled by the use of quinoline-based ligands [26]. Another example for ligand-enabled g-C(sp3)-H arylation was reported by Yu et al. They showed that mono-N-protected amino acid ligands could enable the cross-coupling between the g-C(sp3)-H bond and arylboron reagents [27].
In 2014,Chen et al. [28] and Shi et al. [29] independently reported the Pd-catalyzed mono-arylation reactions of alanine derivatives by using an 8-aminoquinoline-type directing group.
These reactions provide an efficient approach for the preparation of various β-arylated-a-amino acids from readily available alanine.The reaction conditions are described in Scheme 10. Chen et al. [28] and Shi et al. [29] also demonstrated the removal of the 8-aminoquinoline auxiliary. Meanwhile,Liu and Zhang reported the Pd-catalyzed β-arylation reaction of proline derivatives again using 8-aminoquinoline as the directing group [30]. They also showed the vinylation and alkylation at C-3 position of the proline derivatives.

|
Download:
|
| Scheme 10.Improved conditions for mono-arylation of alanine derivatives by using an 8-aminoquinoline directing group. TCE = Trichloroethylene. | |
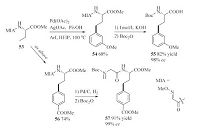
Efficient directing group designed at the N-terminus of amino acids make possible for the g-arylation reactions. For example,Ma et al. developed the 2-methoxyiminoacetyl (MIA) group as the directing and protecting group for the N-atom in the process [31]. A wide range of synthetically important functional groups survived in this transformation. Meanwhile,the MIA directing group could be readily removed or converted to other moieties (Scheme 11).
|
Download:
|
| Scheme 11.Pd-catalyzed MIA-directed g-C(sp3)-H arylation. PivOH = pivalic acid.HFIP = hexafluoroisopropanol. | |
Besides,Carretero et al. [32] and Chen et al. [33] independently accomplished the g-arylation reactions of amino acid derivatives with N-(2-pyridyl)sulfonyl and N-picolinoyl (PA) directing groups.The transition metal-catalyzed C-H functionalization reactions can be applied to the synthesis of more complex target compounds.For example,Yu et al. accomplished the post-synthetic modification of some peptides through Pd-catalyzed C(sp3)-H arylation reaction.They showed that site-selective C(sp3)-H arylation can occur at the amino acid N-terminus in di-,tri-,and even tetra-peptides [14]. In these reactions the native amino acid moiety in the peptides reacted as the ligand (Scheme 12).
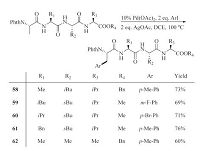
|
Download:
|
| Scheme 12.Pd-catalyzed C(sp3)–H arylation of tetrapeptides. | |
Meanwhile,the new C-H functionalization reactions for the synthesis of unnatural amino acids have also been applied to the total synthesis of some natural products [34]. For example,Chen et al. reported the total synthesis of Hibispeptins A,in which the key Ile-Hpa pseudodipeptide motif was synthesized through Pdcatalyzed g-C(sp3)-H arylation reaction [34b]. It is worth mentioning that a new directing group PR (pyridylmethylaminebased directing group) was developed for this reaction (Scheme 13). It is important to point out that arylation of a-C(sp3)-H were also widely exploited for the synthesis or modification of amino acids [35].

|
Download:
|
| Scheme 13.Pd-catalyzed C(sp3)-H arylation for the synthesis of key motif in Hibispeptins A. | |
Compared with the arylation reactions of the C(sp3)-H bonds of amino acid derivatives,the related vinylation and alkynylation reactions were lagged far behind. In 2011,Chen et al. reported the PA-directed g-position C(sp3)-H vinylation of the cyclohexyl amino acid with 1-iodocyclohept-1-ene with a moderate yield as an isolated example [33]. Later in 2014,Chen et al. reported a general Pd-catalyzed N-quinolyl carboxamide-directed vinylation reaction of β-position C(sp3)-H bond of protected alanine [36]. In the presence of Pd(OAc)2,AgOAc,and TFA in 1,4-dioxane,a wide range of multi-substituted vinyl iodides could be readily coupled with alanine derivatives (Scheme 14). More recently,Chen et al.further developed Pd-catalyzed alkynylation reaction of the methylene C(sp3)-H bonds in amino acids [37]. This reaction provides good access to β-alkynyl a-amino acids through the coupling of the amino acid precursors with alkynyl bromide.

|
Download:
|
| Scheme 14.Pd-catalyzed 8-aminoquinoline directed C(sp3)-H vinylation. | |
In 2012,Daugulis et al. reported the alkylation of C(sp3)-H bonds in amino acid derivatives [22]. In the present of Pd(OAc)2,Cs3PO4 and CsOPiv,alanine derivative 70 was converted to compound 71 in 42% yield. This reaction was the first example for direct alkylation of C(sp3)-H bonds of the amino acid side chain (Scheme 15).

|
Download:
|
| Scheme 15.Pd-catalyzed 8-aminoquinoline directed alkylation of β-C(sp3)-H in alanine derivative. | |
In 2013,Chen et al. reported the Pd-catalyzed PA-directed alkylation reaction of g-C(sp3)-H bonds [38]. This reaction provides an efficient and straightforward access to N-containing products (Scheme 16).

|
Download:
|
| Scheme 16.Pd-catalyzed PA-directed alkylation of g-C(sp3)-H. | |
Based on the early work of Daugulis et al. [22] on the alkylation of methyl C(sp3)-H bonds,the groups of Chen [39] and Shi [40] independently developed the alkylation reactions of the methylene C(sp3)-H bonds. Chen et al. accomplished secondary C(sp3)-H alkylation in the presence of (BnO)2PO2H and Ag2CO3. However,their reaction scope was limited to activated alkyl halides such as MeI and a-iodoacetate. In Chen’s work,(BnO)2PO2H played the roles as phase-transfer-catalyst for Ag salts and ligand in the oxidation addition and reductive elimination steps. Pd(Ⅱ)/Pd(Ⅳ) manifold (Fig. 5) was suggested for Pd-catalyzed C(sp3)-H alkylation reactions [7a,23c]. The mechanism for the oxidation addition step remained unclear. Chen and co-workers proposed that the reaction might proceed through an SN2 pathway,although the radical-type or Pd(Ⅲ) pathway [24] could not be excluded.
|
Download:
|
| Fig. 5.Proposed mechanism for Pd-catalyzed 8-aminoquinoline directed C(sp3)-H alkylation reaction. | |
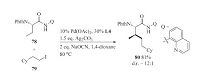
Note that the scope limitation of the alkyl electrophiles was partially overcome by Shi et al. in 2014 [41]. Shi et al. found that NaOCN and 4-Cl-C6H4SO2NH2 (L4) could promote the coupling of un-activated methylene C(sp3)-H bonds with un-activated primary alkyl iodides (Scheme 17).
|
Download:
|
| Scheme 17.Pd-catalyzed 8-aminoquinoline directed alkylation of un-activated methylene C(sp3)-H with un-activated alkyl iodides. | |
Recent developments of the primary and secondary C(sp3)-H bond functionalization reactions have been employed for the selective modification of natural amino acids and even peptides.
The side chain of amino acids were successfully modified through sequential C-H activation and C-X (X = C,B,O,N,F,etc.) bond formation reactions. Although considerable progress has been achieved in this field,there still remain several challenges.
First,the reactivity is still low for un-activated C(sp3)-H bonds,especially the secondary C(sp3)-H bonds. More efforts are needed to solve this problem by strategic designing of new ligands and directing groups [42]. Second,the directing groups (e.g. 8- aminoquinoline) and protecting groups (e.g. Phth) are not common in the field of amino acids or peptide/protein chemistry. These protecting and directing groups need additional steps to introduce and then remove,which decreases the atom and step efficiency of the modification process. Third,cysteine is not a good substrate in the C-H functionalization reactions at present. As thiol-containing amino acids are of importance for protein chemical synthesis,it would be important to develop conditions for the modification of cysteine derivatives. Fourth,although palladium is usually used for the C-H functionalization of aliphatic C-H bonds,other transition metal catalysis systems (for instance,Fe and Cu) [43] and even visible light catalyst [44] should also be developed for the modification of the C-H bonds of amino acids.
Notwithstanding the above current challenges,with the rapid expansion of the repertoire of the C-H functionalization methodology,we expect that C-H functionalization will become a costeffective approach for the synthesis of many unnatural amino acids.
| [1] | (a) A.B. Hughes, Amino Acids, Peptides and Proteins in Organic Chemistry Analysis and Function of Amino Acids and Peptides, John Wiley & Sons, 2013;(b) Y. Lin, J. Wang, Y. Lu, Functional tuning and expanding of myoglobin by rational protein design, Sci. China Chem. 57(2014) 346-355;(c) Y. Huang, L. Liu, Chemical synthesis of crystalline proteins, Sci. China Chem. 58(2015) 1779-1781. |
| [2] | F. Albericio, H.G. Kruger, Therapeutic peptides, Future Med. Chem. 4(2012) 1527-1531. |
| [3] | (a) J. Seyden-Penne, Chiral Auxiliaries and Ligands in Asymmetric Synthesis, Wiley-(Ⅰ)nterscience, 1995;(b) M.Y. Liu, S.B. Hong, W. Zhang, W. Deng, Expedient copper-catalyzed borylation reactions using amino acids as ligands, Chin. Chem. Lett. 26(2015) 373-376;(c) J. Kim, M. Sim, N. Kim, S. Hong, Asymmetric C-H functionalization of cyclopropanes using an isoleucine-NH2 bidentate directing group, Chem. Sci. 6(2015) 3611-3616. |
| [4] | (a) X. Lu, J. Yi, Z.Q. Zhang, et al., Expedient synthesis of chiral α-amino acids through nickel-catalyzed reductive cross-coupling, Chem. Eur. J. 20(2014) 15339-15343;(b) C.Z. Tao, Z.T. Zhang, J.W. Wu, R.H. Li, Z.L. Cao, Synthesis of unnatural Nglycosyl α-amino acids via Petasis reaction, Chin. Chem. Lett. 25(2014) 532-534;(c) X.B. Wang, X.L. Wang, J. Hu, et al., Study on the synthesis of novel sugar amino acids, Acta Chim. Sin. 73(2015) 699-704;(d) F. Ni, C. Fu, X. Gao, et al., N-phosphoryl amino acid models for P-N bonds in prebiotic chemical evolution, Sci. China Chem. 58(2015) 374-382. |
| [5] | C. Ná jera, J.M. Sansano, Catalytic asymmetric synthesis of α-amino acids, Chem. Rev. 107(2007) 4584-4671. |
| [6] | G.T. Notte, T. Sammakia, Kinetic resolution of protected α-amino acid derivatives by a chiral O-nucleophilic acyl transfer catalyst, J. Am. Chem. Soc. 128(2006) 4230-4231. |
| [7] | (a) L. Ackermann, Carboxylate-assisted transition-metal-catalyzed C-H bond functionalizations:mechanism and scope, Chem. Rev. 111(2011) 1315-1345;(b) X. Chen, K.M. Engle, D.H. Wang, J.Q. Yu, Palladium(Ⅱ)-catalyzed C-H activation/C-C cross-coupling reactions:versatility and practicality, Angew. Chem. (Ⅰ)nt. Ed. 48(2009) 5094-5115;(c) K.M. Engle, T.S. Mei, M. Wasa, J.Q. Yu, Weak coordination as a powerful means for developing broadly useful C-H functionalization reactions, Acc. Chem. Res. 45(2012) 788-802;(d) R. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer, O. Baudoin, Functionalization of organic molecules by transition-metal-catalyzed C(sp3)-H activation, Chem. Eur. J. 16(2010) 2654-2672;(e) (Ⅰ).A.(Ⅰ). Mkhalid, J.H. Barnard, T.B. Marder, J.M. Murphy, J.F. Hartwig, C-H activation for the construction of C-B bonds, Chem. Rev. 110(2010) 890-931;(f) S.R. Neufeldt, M.S. Sanford, Controlling site selectivity in palladium-catalyzed C-H bond functionalization, Acc. Chem. Res. 45(2012) 936-946;(g) X.X. Qi, P.H. Chen, G.S. Liu, Advances and challenges in palladium-catalyzed intermolecular selective allylic C-H functionalization of alkenes, Sci. China Chem. 58(2015) 1249-1251;(h) Y. Rao, G. Shan, X.L. Yang, Some recent advances in transition-metal-catalyzed ortho sp2 C-H functionalization using Ru, Rh, and Pd, Sci. China Chem. 57(2014) 930-944;(i) J.B. Wang, Gold-catalyzed chemo-and site-selective direct C-H functionalization of phenols with diazo compounds, Sci. China Chem. 57(2014) 1057;(j) K.H. Wang, F.D. Hu, Y. Zhang, J.B. Wang, Directing group-assisted transitionmetal-catalyzed vinylic C-H bond functionalization, Sci. China Chem. 58(2015) 1252-1265;(k) Y.H. Yang, C.Y. Wang, Direct silylation reactions of inert C-H bonds via transition metal catalysis, Sci. China Chem. 58(2015) 1266-1279. |
| [8] | (a) D.Y.K. Chen, S.W. Youn, C-H activation:a complementary tool in the total synthesis of complex natural products, Chem. Eur. J. 18(2012) 9452-9474;(b) A.F.M. Noisier, M.A. Brimble, C-H functionalization in the synthesis of amino acids and peptides, Chem. Rev. 114(2014) 8775-8806. |
| [9] | B.V.S. Reddy, L.R. Reddy, E.J. Corey, Novel acetoxylation and C-C coupling reactions at unactivated positions in α-amino acid derivatives, Org. Lett. 8(2006) 3391-3394. |
| [10] | (a) R. Giri, J. Liang, J.G. Lei, et al., Pd-catalyzed stereoselective oxidation of methyl groups by inexpensive oxidants under mild conditions:a dual role for carboxylic anhydrides in catalytic C-H bond oxidation, Angew. Chem. (Ⅰ)nt. Ed. 44(2005) 7420-7424;(b) L.V. Desai, H.A. Malik, M.S. Sanford, Oxone as an inexpensive, safe, and environmentally benign oxidant for C-H bond oxygenation, Org. Lett. 8(2006) 1141-1144;(c) V.G. Zaitsev, D. Shabashov, O. Daugulis, Highly regioselective arylation of sp3 C-H bonds catalyzed by palladium acetate, J. Am. Chem. Soc. 127(2005) 13154-13155. |
| [11] | S.Y. Zhang, G. He, Y. Zhao, et al., Efficient alkyl ether synthesis via palladiumcatalyzed, picolinamide-directed alkoxylation of unactivated C(sp3)-H and C(sp2)-H bonds at remote positions, J. Am. Chem. Soc. 134(2012) 7313-7316. |
| [12] | K. Chen, S.Q. Zhang, H.Z. Jiang, J.W. Xu, B.F. Shi, Practical synthesis of anti-bhydroxy-α-amino acids by Pd(Ⅱ)-catalyzed sequential C(sp3)-H functionalization, Chem. Eur. J. 21(2015) 3264-3270. |
| [13] | (a) Q.Q. He, G.M. Fang, L. Liu, Design of thiol-containing amino acids f or native chemical ligation at non-Cys sites, Chin. Chem. Lett. 24(2013) 265-269;(b) H. Liu, X. Li, Synthesis of highly-strained cyclic tetrapeptides via peptide hydrazide based ligation, Acta Chim. Sin. 72(2014) 1197-1198;(c) G.M. Fang, J.X. Wang, L. Liu, Convergent chemical synthesis of proteins by ligation of peptide hydrazides, Angew. Chem. (Ⅰ)nt. Ed. 51(2012) 10347-10350;(d) G.M. Fang, Y.M. Li, F. Shen, et al., Protein chemical synthesis by ligation of peptide hydrazides, Angew. Chem. (Ⅰ)nt. Ed. 50(2011) 7645-7649. |
| [14] | W. Gong, G. Zhang, T. Liu, R. Giri, J.Q. Yu, Site-selective C(sp3)-H functionalization of di-, tri-, and tetrapeptides at the N-terminus, J. Am. Chem. Soc. 136(2014) 16940-16946. |
| [15] | G. He, Y. Zhao, S. Zhang, C. Lu, G. Chen, Highly efficient syntheses of azetidines, pyrrolidines, and indolines via palladium catalyzed intramolecular amination of C(sp3)-H and C(sp2)-H bonds at γ and δ positions, J. Am. Chem. Soc. 134(2012) 3-6. |
| [16] | G. He, S.Y. Zhang, W.A. Nack, Q. Li, G. Chen, Use of a readily removable auxiliary group for the synthesis of pyrrolidones by the palladium-catalyzed intramolecular amination of unactivated γ C(sp3)-H bonds, Angew. Chem. (Ⅰ)nt. Ed. 52(2013) 11124-11128. |
| [17] | Q. Zhang, K. Chen, W. Rao, et al., Stereoselective synthesis of chiral α-amino-blactams through palladium(Ⅱ)-catalyzed sequential monoarylation/amidation of C(sp3)-H bonds, Angew. Chem. (Ⅰ)nt. Ed. 52(2013) 13588-13592. |
| [18] | L.S. Zhang, G. Chen, X. Wang, et al., Direct borylation of primary C-H bonds in functionalized molecules by palladium catalysis, Angew. Chem. (Ⅰ)nt. Ed. 53(2014) 3899-3903. |
| [19] | R.Y. Zhu, K. Tanaka, G.C. Li, et al., Ligand-enabled stereoselective β-C(sp3)-H fluorination:synthesis of unnatural enantiopure anti-β-fluoro-α-amino acids, J. Am. Chem. Soc. 137(2015) 7067-7070. |
| [20] | Q. Zhang, X.S. Yin, K. Chen, S.Q. Zhang, B.F. Shi, Stereoselective synthesis of chiral β-fluoro α-amino acids via Pd(Ⅱ)-catalyzed fluorination of unactivated methylene C(sp3)-H bonds:scope and mechanistic studies, J. Am. Chem. Soc. 137(2015) 8219-8226. |
| [21] | J. Miao, K. Yang, M. Kurek,H. Ge, Palladium-catalyzed site-selective fluorination of unactivated C(sp3)-H bonds, Org. Lett. 17(2015) 3738-3741. |
| [22] | L.D. Tran, O. Daugulis, Nonnatural amino acid synthesis by using carbon-hydrogen bond functionalization methodology, Angew. Chem. (Ⅰ)nt. Ed. 51(2012) 5188-5191. |
| [23] | (a) D. Shabashov, O. Daugulis, Auxiliary-assisted palladium-catalyzed arylation and alkylation of sp2 and sp3 carbon-hydrogen bonds, J. Am. Chem. Soc. 132(2010) 3965-3972;(b) L.M. Xu, B.J. Li, Z. Yang, Z.J. Shi, Organopalladium((Ⅰ)V) chemistry, Chem. Soc. Rev. 39(2010) 712-733;(c) A.J. Hickman, M.S. Sanford, High-valent organometallic copper and palladium in catalysis, Nature 484(2012) 177-185. |
| [24] | (a) D.C. Powers, M.A.L. Geibel, J.E.M.N. Klein, T. Ritter, Bimetallic palladium catalysis:direct observation of Pd(Ⅲ)-Pd(Ⅲ) intermediates, J. Am. Chem. Soc. 131(2009) 17050-17051;(b) N.R. Deprez, M.S. Sanford, Synthetic and mechanistic studies of Pd-catalyzed C-H arylation with diaryliodonium salts:evidence for a bimetallic high oxidation state Pd intermediate, J. Am. Chem. Soc. 131(2009) 11234-11241. |
| [25] | J. He, S. Li, Y. Deng, et al., Ligand-controlled C(sp3)-H arylation and olefination in synthesis of unnatural chiral α-amino acids, Science 343(2014) 1216-1220. |
| [26] | J. He, R. Takise, H. Fu, J.Q. Yu, Ligand-enabled cross-coupling of C(sp3)-H bonds with arylsilanes, J. Am. Chem. Soc. 137(2015) 4618-4621. |
| [27] | K.S. Chan, M. Wasa, L. Chu, et al., Ligand-enabled cross-coupling of C(sp3)-H bonds with arylboron reagents via Pd(Ⅱ)/Pd(0) catalysis, Nat. Chem. 6(2014) 146-150. |
| [28] | B. Wang, W.A. Nack, G. He, S.Y. Zhang, G. Chen, Palladium-catalyzed trifluoroacetate-promoted mono-arylation of the β-methyl group of alanine at room temperature:synthesis of β-arylated α-amino acids through sequential C-H functionalization, Chem. Sci. 5(2014) 3952-3957. |
| [29] | K. Chen, S.Q. Zhang, J.W. Xu, F. Hu, B.F. Shi, A general and practical palladiumcatalyzed monoarylation of β-methyl C(sp3)-H of alanine, Chem. Commun. 50(2014) 13924-13927. |
| [30] | R. Feng, B. Wang, Y. Liu, Z. Liu, Y. Zhang, Efficient synthesis of cis-3-substituted prolines by bidentate-assisted palladium catalysis, Eur. J. Org. Chem. 2015(2015) 142-151. |
| [31] | M. Fan, D. Ma, Palladium-catalyzed direct functionalization of 2-aminobutanoic acid derivatives:application of a convenient and versatile auxiliary, Angew. Chem. (Ⅰ)nt. Ed. 52(2013) 12152-12155. |
| [32] | N. Rodriguez, J.A. Romero-Revilla, M.A. Fernandez-(Ⅰ)banez, J.C. Carretero, Palladium-catalyzed N-(2-pyridyl)sulfonyl-directed C(sp3)-H g-arylation of amino acid derivatives, Chem. Sci. 4(2013) 175-179. |
| [33] | G. He, G. Chen, A practical strategy for the structural diversification of aliphatic scaffolds through the palladium-catalyzed picolinamide-directed remote functionalization of unactivated C(sp3)-H bonds, Angew. Chem. (Ⅰ)nt. Ed. 50(2011) 5192-5196. |
| [34] | (a) Y. Feng, G. Chen, Total synthesis of celogentinjC by stereoselective C-H activation, Angew. Chem. (Ⅰ)nt. Ed. 49(2010) 958-961;(b) G. He, S.Y. Zhang, W.A. Nack, et al., Total synthesis of Hibispeptin A via Pdcatalyzed C(sp3)-H arylation with sterically hindered aryl iodides, Org. Lett. 16(2014) 6488-6491. |
| [35] | (a) X.H. Wei, G.W. Wang, S.D. Yang, Enantioselective synthesis of arylglycine derivatives by direct C-H oxidative cross-coupling, Chem. Commun. 51(2015) 832-835;(b) F. Bellina, R. Rossi, Transition metal-catalyzed direct arylation of substrates with activated sp3-hybridized C-H bonds and some of their synthetic equivalents with aryl halides and pseudohalides, Chem. Rev. 110(2010) 1082-1146;(c) K.Z. Li, Q. Wu, J.B. Lan, J.S. You, Coordinating activation strategy for C(sp3)-H/C(sp3)-Hcross-couplingtoaccessβ-aromatica-aminoacids,Nat.Commun.6(2015). |
| [36] | B. Wang, C. Lu, S.Y. Zhang, et al., Palladium-catalyzed stereoretentive olefination of unactivated C(sp3)-H bonds with vinyl iodides at room temperature:synthesis of β-vinyl α-amino acids, Org. Lett. 16(2014) 6260-6263. |
| [37] | B. Wang, G. He, G. Chen, Synthesis of β-alkynyl α-amino acids via palladiumcatalyzed alkynylation of unactivated C(sp3)-H bonds, Sci. China Chem. 58(2015) 1345-1348. |
| [38] | S.Y. Zhang, G. He, W.A. Nack, et al., Palladium-catalyzed picolinamide-directed alkylation of unactivated C(sp3)-H bonds with alkyl iodides, J. Am. Chem. Soc. 135(2013) 2124-2127. |
| [39] | S.Y. Zhang, Q. Li, G. He, W.A. Nack, G. Chen, Stereoselective synthesis of β-alkylated α-amino acids via palladium-catalyzed alkylation of unactivated methylene C(sp3)-H bonds with primary alkyl halides, J. Am. Chem. Soc. 135(2013) 12135-12141. |
| [40] | K. Chen, F. Hu, S.Q. Zhang, B.F. Shi, Pd(Ⅱ)-catalyzed alkylation of unactivated C(sp3)-H bonds:efficient synthesis of optically active unnatural α-amino acids, Chem. Sci. 4(2013) 3906-3911. |
| [41] | K. Chen, B.F. Shi, Sulfonamide-promoted palladium(Ⅱ)-catalyzed alkylation of unactivated methylene C(sp3)-H bonds with alkyl iodides, Angew. Chem. (Ⅰ)nt. Ed. 53(2014) 11950-11954. |
| [42] | (a) K. Chen, Z.W. Li, P.X. Shen, H.W. Zhao, Z.J. Shi, Development of modifiable bidentate amino oxazoline directing group for Pd-catalyzed arylation of secondary C-H bonds, Chem. Eur. J. 21(2015) 7389-7393;(b) G. Rouquet, N. Chatani, Catalytic functionalization of C(sp2)-H and C(sp3)-H bonds by using bidentate directing groups, Angew. Chem. (Ⅰ)nt. Ed. 52(2013) 11726-11743. |
| [43] | (a) R. Shang, L. (Ⅰ)lies, E. Nakamura, (Ⅰ)ron-catalyzed directed C(sp2)-H and C(sp3)-H functionalization with trimethylaluminum, J. Am. Chem. Soc. 137(2015) 7660-7663;(b) Q. Zhang, Y. Lv, Y. Li, T. Xiong, Q. Zhang, Copper-catalyzed benzylic sp3 C-H amination reaction of amidines:synthesis of quinazoline derivatives, Acta Chim. Sin. 72(2014) 1139-1143. |
| [44] | X.W. Gao, Q.Y. Meng, J.X. Li, et al., Visible light catalysis assisted site-specific functionalization of amino acid derivatives by C-H bond activation without oxidant:cross-coupling hydrogen evolution reaction, ACS Catal. 5(2015) 2391-2396. |
 2016, Vol.27
2016, Vol.27 


