b Department of Pharmaceutical Chemistry, Y. B. Chavan College of Pharmacy, Dr. Rafiq Zakaria Campus, Aurangabad 431001, India
In recent years,the incidence of systemic fungal infection is increasing significantly due to an increase in number of patients undergoing organ transplants,anticancer chemotherapy and patients with AIDS. Commonly used azole antifungal agents are fluconazole,itraconazole,miconazole and voriconazole displayed broad spectrum antifungal activity [1]. Azoles have broad spectrum activities against most yeasts and filamentous fungi and are the drug of choice for antifungal chemotherapy [2]. These antifungal drugs inhibiting CYP51 in the process of biosynthesis of ergosterol through a mechanism in which the heterocyclic nitrogen atom (N-4 of triazole) binds to the heme iron atom [3]. However,increasing the use of these antifungal drugs has led to increase in resistance to these drugs [4, 5, 6]. Hence,there is an urgent need for the development of more potent,broad spectrum antifungal agents with fewer side effects and improved efficacy to cure fungal infections.
Coumarin and their derivatives have attracted much more considerable attention due to their extensive biological activities. In recent years,studies have shown that coumarin incorporated with some nitrogen-containing heterocyclic moieties; viz. azetidine,thiazolidine,thiazole and oxadiazole were not only significantly increases the antimicrobial efficiency but also broadens their antimicrobial spectrum [7, 8]. Antioxidants play a vital role in the body defense mechanism by regulating the generation and elimination of reactive oxygen species (ROS) such as hydroxyl radicals,superoxide radicals,singlet oxygen and hydrogen peroxide radicals those generated from excessive oxidative stress and normal metabolic activities. The regulating mechanism includes detoxification of excess ROS,if not,the high concentrations of free radical damages the normal cell structures,embedded proteins,lipids,carbohydrates and also damages the nitrogen bases of nucleic acids leading to mutations and also causes cancer,aging and neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases. In addition to the body’s defense mechanism includes superoxide dismutase (SOD),catalase and glutathione peroxidase,antioxidants also regulate the concentration of ROS by interacting with them and prevent their influence on other molecules. Thus,the discovery and development of novel synthetic radical scavengers attained an immense importance in organic chemistry. Many coumarin derivatives have unique ability to scavenge reactive oxygen species (ROS) free radicals,such as hydroxyl,superoxide radicals or hypochlorous acid and to influence processes involving free radical injury [9, 10]. Coumarin derivatives exhibit enormous biological activities such as,antimicrobial,anti-HIV,antibiotic,anticancer,muscle relaxant,anti-inflammatory and anticoagulant activity [11]. The 1,2,3-triazole-based compounds are reported to possess a wide range of biological activities such as antifungal [12],antitubercular [13],antiallergic,antibacterial,anti-HIV activity [14],α-glycosidase inhibitor [15],antimicrobial [16],anticoccidiostats [17],anticonvulsant and antitumor [18],antimalarial [19],antiviral [20] and antimycobacterial activity [21]. Triazole has been used to improve the pharmacokinetic properties of the desired drug [22].
In recent years,a library of coumarin derivatives conjugated with 1,2,3-triazole moiety were synthesized and proved to possess antifungal activity. Therefore,the incorporation of triazole moiety is essential for the enhancement of activity [23]. Coumarin-based triazole 1 (Fig. 1) displays antifungal activity against three fungal strain viz. Candida albicans,Saccharomyces cerevisiae and Aspergillus fumigatus [23]. Similarly,2H-chromen-2-one derivative 2,decorated with 1,2,3-triazole moiety exhibits antifungal activity against four fungal strains,viz. Aspergillus niger,A. fumigates,Aspergillus flavus and C. albicans [24] and 3-[1-(4,5-dicarbomethoxy- 1,2,3-triazoloacetyl)]coumarin 3 displays good antifungal activity against the fungal strain A. niger [25] (Fig. 1).
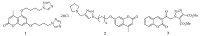
|
Download:
|
| Fig. 1.Coumarin-triazole conjugates 1-3 displays antifungal activity. | |
In continuation of our earlier work [26] on synthesis and biological properties of heterocyclic moieties and the importance of coumarin and 1,2,3-triazole moieties as a single molecular scaffold,herein we would like to report the design and syntheses of new coumarin-linked triazole hybrids. The coumarin derivatives have been well reported for antioxidant activity and 1,2,3-triazole ring is good scaffold for antifungal activity. Thus,we have evaluated the synthesized compounds for their antifungal and antioxidant activities. The computational parameters like docking study for antifungal activity and ADME prediction of synthesized coumarin-triazole conjugates 8a-h were also performed.
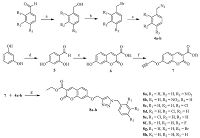
2. Experimental 2.1. ChemistryAll the solvents and reagents were purchased from commercial suppliers Spectrochem Pvt. Ltd.,Sigma Aldrich and Rankem India Ltd. used without further purification. The progress of each reaction was monitored by ascending thin layer chromatography (TLC) using TLC aluminum sheets,silica gel F254 precoated,Merck,Germany and locating the spots using UV light as the visualizing agent or iodine vapors. Melting points were taken in open capillary method and are uncorrected. 1H NMR spectra were recorded (CDCl3/DMSO-d6) on Bruker Avance 400 MHz NMR spectrometer. 13C NMR spectra were recorded (DMSO-d6) on Bruker Avance100 MHz NMR spectrometer. Chemical shifts (δ) are reported in parts per million (ppm) using tetramethylsilane (TMS) as an internal standard. The splitting pattern abbreviations are designed as singlet (s); doublet (d); double doublet (dd); bs (broad singlet),bd (broad doublet),triplet (t); quartet (q) and multiplet (m). Bruker Daltonics MicroTOF-Q-II with electron spray ionization (ESI) was used for HRMS data. The synthetic protocol employed for the synthesis of ethyl 7-((1-(benzyl)-1H-1,2,3-triazol-4-yl)methoxy)- 2-oxo-2H-chromene-3-carboxylates has been presented in Scheme 1.
|
Download:
|
| Scheme 1.Synthetic route for target compounds 8a-h. Reagents and conditions: (a) NaBH4, methanol, 0 8C to r.t., 2 h; (b) PBr3, DCM, 0 8C, 0.5 h; (c) NaN3, acetone-H2O (3:1), rt, 24 h; (d) (i) POCl3, DMF, CH3CN, 0 8C, 3 h; (ii) H2O, 50 8C, 2 h; (e) Diethyl malonate, H2SO4, 0 8C, 6 h; (f) Propargyl bromide, K2CO3, DMF, 3 h; (g) Cu(OAc)2 (20 mol.%), t- BuOH-H2O (3:1), rt, 24-36 h. | |
General procedure for the synthesis of ethyl 7-((1-(benzyl)- 1H-1,2,3-triazol-4-yl)methoxy)-2-oxo-2H-chromene-3-carboxylates 8a-h: To the solution of ethyl 2-oxo-7-(prop-2-yn-1- yloxy)-2H-chromene-3-carboxylate (7) (0.5 mmol),substituted benzyl azide 4a-h (0.5 mmol) and copper diacetate (Cu(OAc)2) (20 mol%) in t-BuOH-H2O (3:1,8 mL) and the resulting mixture was stirred at room temperature for 24-36 h. The progress of the reaction was monitored by TLC using ethyl acetate:hexane as a solvent system. The reaction mixture was quenched with crushed ice and extracted with ethyl acetate (2 × 25 mL). The organic extracts were washed with brine solution (2 × 25 mL) and dried over anhydrous sodium sulphate. The solvent was evaporated under reduced pressure to afford the corresponding crude compounds. The obtained crude compounds were crystallized using ethanol.
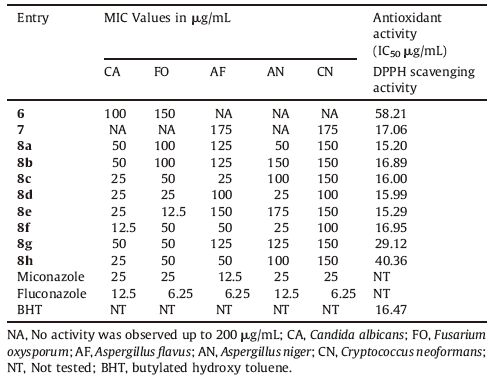
2.2. Biological activity 2.2.1. Antifungal activityThe antifungal activity was evaluated against five human pathogenic fungal strains,such as C. albicans (NCIM3471),Fusarium oxysporum (NCIM1332),A. flavus (NCIM539),A. niger (NCIM1196) and Cryptococcus neoformans (NCIM576),which are often encountered clinically and were compared with standard drug miconazole. Minimum inhibitory concentration (MIC) values were determined using standard agar method [27].
2.2.2. Antioxidant activityIn the present study,antioxidant activity of the synthesized compounds has been assessed in vitro by the 1,1-diphenyl-2- picrylhydrazyl (DPPH) radical scavenging assay [28] and the results were comparedwith standard synthetic antioxidant BHT (Butylated Hydroxy Toluene). The hydrogen atom or electron donation ability of the compounds was measured from the bleaching of the purple coloredmethanol solutionof1,1-diphenyl-1-picrylhydrazyl(DPPH). The spectrophotometric assay uses the stable radical DPPH as a reagent. 1 mL of various concentrations of the test compounds (5,10,25,50 and 100 μg/mL) in methanol was added to 4mL of 0.004% (w/v) methanol solution of DPPH. After a 30 min incubation period at room temperature,the absorbance was measured against blank at 517 nm. The percent inhibition (I %) of free radical production from DPPH was calculated by the following equation.
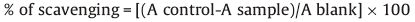
Where ‘A control’ is the absorbance of the control reaction (containing all reagents except the test compound) and ‘A sample’ is the absorbance of the test compound. Tests were carried at in triplicate.
2.3. Computational study 2.3.1. Molecular dockingThe 3D model structure of cytochrome P450 lanosterol 14α-demethylase of C. albicans was built using homology modeling. Amino acid sequence of enzyme was obtained from the Universal Protein Resource (http://www.uniprot.org/) (Accession Code: P10613) and sequence homologous was obtained from Protein Data Bank (PDB) using Blast search. Based on the result of blast search,we used the crystal structure of human lanosterol 14α-demethylase (CYP51) with azole (PDB ID:3LD6) as a template for homology modeling. The VLifeMDS 4.3 ProModel was used for modeling of the 3D structure of protein based on the amino acid sequences of a close homologue. Alignment of amino acid sequence of CA-CYP51 (P10613) and human CYP51 (3LD6_B) is shown in Fig. S1 in Supporting information. The Blosum-62 matrix was used with a gap penalty of 1. The model was then energy minimized using the MMFF94 force field [29]. Manual inspection was made to ensure the conserved motifs and loops were correctly aligned. The quality of generated C. albicans lanosterol 14α-demethylase model was assessed by using the well-validated program likes PROCHECK [30] and its structural validation is shown in Fig. S2 in Supporting information. The further structural superimposition was performed to know the structural coordinate of target protein and RMSD value was found within standard range of 0.997607Å . The molecular docking study of the synthesized compounds 8a-h and standard drugs fluconazole and miconazole were performed against homology built cytochrome P450 lanosterol 14α-demethylase of C. albicans to understand the binding interactions using VLife MDS 4.3 package following standard procedures [31].
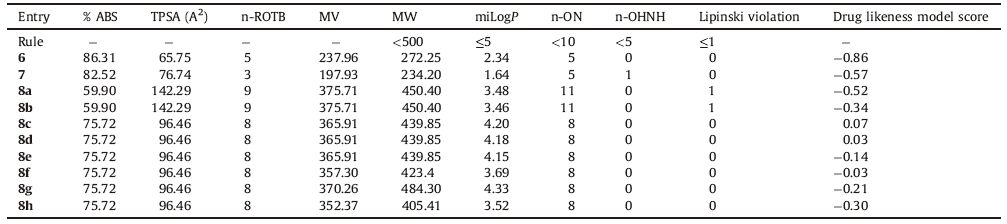
2.3.2. ADME propertiesThe success of a drug is determined not only by good efficacy but also by an acceptable ADME (absorption,distribution,metabolism and excretion) profile. In this study,we calculated molecular volume (MV),molecular weight (MW),logarithm of partition coefficient (miLogP),number of hydrogen bond acceptors (n-ON),number of hydrogen bonds donors (n-OHNH),topological polar surface area (TPSA),number of rotatable bonds (n-ROTB) and Lipinski’s rule of five [32] using Molinspiration online property calculation toolkit [33]. Absorption (% ABS) was calculated by: % ABS = 109-(0.345 × TPSA) [34]. Drug-likeness model score (a collective property of physic-chemical properties,pharmacokinetics and pharmacodynamics of a compound is represented by a numerical value) was computed by MolSoft software [35].
3. Results and discussion 3.1. ChemistryWe have described the syntheses of a series of new ethyl-7-((1- (benzyl)-1H-1,2,3-triazol-4-yl)methoxy)-2-oxo-2H-chromene-3- carboxylates 8a-h as potential antifungal and antioxidant agents from commercially available starting materials. These compounds 8a-h were formed by the fusion of benzyl azides and coumarinbased alkyne via click chemistry approach. The benzyl azides 4a-h has been prepared from the corresponding benzaldehydes via NaBH4 reduction,bromination and nucleophilic substitution reaction of sodium azide according to the reported procedure [36] (Scheme 1). Synthesis of 2,4-dihydroxybenzaldehyde 5 was achieved from resorcinol by Vilsmeier-Haack reaction according to the literature procedure [37]. The synthesis of ethyl-7-hydroxy-2- oxo-2H-chromene-3-carboxylate 6 has been achieved via Pechmann condensation between 2,4-dihydroxybenzaldehyde 5 and diethylmalonate in the presence of acid [38] in 80% yield (Scheme 1). The treatment of compound 6 with propargyl bromide in presence of K2CO3 as a base in N,N-dimethylformamide (DMF) at room temperature afforded ethyl-2-oxo-7-(prop-2-yn-1-yloxy)- 2H-chromene-3-carboxylate 7 in 95% yield (Scheme 1).
Finally,benzylazides 4a-h and coumarin-based alkyne 7,on 1,3-dipolar cycloaddition reaction in t-BuOH-H2O (3:1) mixture and catalytic amount of copper diacetate Cu(OAc)2 at room temperature for 24-36 h afforded the corresponding regioselective 1,4-disubstituted-1,2,3-triazole incorporated coumarin derivatives 8a-h in quantitative isolated yield (88%-93%) (Scheme 1).
The regioselective formation of 1,4-disubstituted 1,2,3-triazolebased coumarin derivatives 8a-h has been confirmed by physical data and spectroscopic methods such as 1H NMR,13C NMR and HRMS. According to the 1H NMR spectrum of representative compound 8b,the triplet at 1.29-1.36 ppm for three protons (methyl group) attached to -OCH2 group of ester present on coumarin ring,the quartet at 4.24-4.35 ppm for two proton attached to methyl group and oxygen heteroatom. The characteristic two singlets at 5.35 and 5.84 ppm were also observed for - OCH2 and -NCH2 protons of triazole derivative 8b,respectively. In addition to this,a sharp singlet observed at 8.47 ppm assigned to triazole ring,thus confirming the regioselective formation of 1,4- disubstituted 1,2,3-triazole ring. Again,the peak was observed at 8.75 ppm,clearly indicates for the proton of coumarin ring. In addition,all the aromatic protons appeared at expected chemical shifts and integral values. The cyclisation of alkyne 7 with 4b to triazole derivative 8b was further confirmed by 13C NMR spectral data,in which the carbon signals of -OCH2 and -NCH2 groups were resonated at 52.3 and 62.3 ppm respectively. The signals at 163.3 and 163.8 indicate the presence of two carbonyl carbon atom,while all other carbons gave peaks at expected values. Again,the formation of compound 8b was confirmed by high resolution mass spectrometry (HRMS). The calculated [M + Na]+ for compound 8b is 473.1073 and observed [M + Na]+ in HRMS at 473.1071. The physical data,yield and time required to complete the reactions and spectroscopic data of compounds are given in supporting information. The proposed structures were confirmed by1H NMR,13C NMR and HRMS (Supporting information). The products were obtained in good yield (88%-93%).
3.2. In vitro antifungal activityThe minimum inhibitory potential of synthesized 1,4-disubstituted 1,2,3-triazole-based coumarin derivatives 8a-h,was evaluated in vitro against five different fungal strains: C. albicans,F. oxysporum,A. flavus,A. niger and C. neoformans strains and results were compared with their precursors 6 and 7 as well as standard drugs miconazole and fluconazole. The MIC values in μg/mL were estimated and the results are summarized in Table 1. The objective of this study was to see the effect of structural transformation on the 7-hydroxycoumarin derivative. The precursors 6 and 7 do not exhibit antifungal activity against all the tested strains. All the synthesized triazole derivatives 8a-h showed many fold enhanced activity when compared with the precursor 6 and 7 against the fungal strain C. albicans. Compound 8f having fluoro- group at para position of phenyl ring has been found to be good inhibitor of C. albicans with MIC values 12.5 μg/mL and two fold active when compared with the standard drug miconazole and equipotent to fluconazole. Compounds 8c (chloro- group at para),8d (chlorogroup at meta),8e (chloro- group at ortho) and 8h with MIC values 25 μg/mL shows equivalent potency for fungal strain C. albicans compared with the standard drug miconazole. While the compounds 8a,8b and 8g with MIC values 50 μg/mL displays less potency against C. albicans. Compound 8e with chloro-group at ortho position of phenyl ring shows two-fold more activity and compound 8d with chloro-group at meta position of phenyl ring shows equivalent potency for fungal strain F. oxysporum compared with the miconazole. Most of the synthesized compounds 8a-h is inactive against the fungal strain A. flavus,A. niger and C. neoformans. However,compound 8d withchloro- group at meta position of phenyl ring and 8f with fluoro- group at para position of phenyl ring shows equivalent activity for fungal strain A. niger when compared with the standard drug miconazole. It is clear from the Table 1,that the incorporation of triazole ring on coumarin derivatives 6 and 7 increases the antifungal activity of the synthesized compounds 8a-h.
|
|
Table 1 In vitro antimicrobial and antioxidant evaluation of coumarin-based triazoles and their precursor molecules. |
All the synthesized compounds 6,7 and 8a-h show good-tomoderate antioxidant activity when compared with the standard drug BHT (Table 1). The antioxidant activity of these compounds may be related to their redox properties,which allow them to act as reducing agents or hydrogen atom donors and scavenge-free radicals. The compounds 8a having nitro- group at para,8c,8d and 8e has chloro- substituent at para,meta and ortho position,respectively,of phenyl ring shows potent activity (IC50 = 15.20,16,15.99 and 15.29 μg/mL,respectively) when compared with the standard drug BHT. The compounds 8g with bromo- group at para position,8h with no substitution on phenyl ring and 6 show less activity when compared with standard drugs.
3.4. Computational studies 3.4.1. Molecular docking study
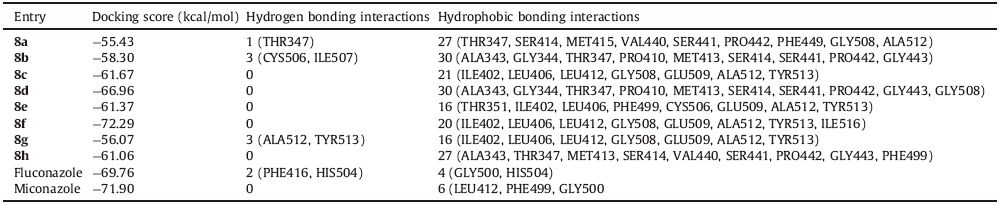
The synthesized compounds 8a-h and standard drugs (fluconazole and miconazole) were docked into the active site of cytochrome P450 lanosterol 14α-demethylase of C. albicans using VLifeMDS 4.3 software package to understand the binding interactions. The docking calculation and hydrogen bond and hydrophobic bond interactions are presented in Table 2. The interaction energy of the compounds 8a-h and their antifungal activity (C. albicans) showed the corresponding results. The most active synthesized compound 8f showed lowest interaction energy that is -72.29 kcal/mol. The standard drugs fluconazole and miconazole have also shown good interaction energy that is -69.76 and -71.90 kcal/mol,respectively. The docking results indicated that the coumarin-triazole core of these synthesized compounds was held in the active pocket by combination of various hydrogen and hydrophobic interactions with cytochrome P450 lanosterol 14a-demethylase. The various hydrophobic interactions occurred between the coumarin-triazole core active site chain of ALA343,GLY344,THR347,THR351,ILE402,LEU406,PRO410,LEU412,MET413,SER414,MET415,VAL440,SER441,
PRO442,GLY443,PHE449,CYS506,GLY508,GLU509,ALA512,TYR513 and ILE516. The amino acid residues such as THR347,CYS506,ILE507 and ALA512 had formed hydrogen bonds with synthesized compounds. The amino acid THR347 had formed hydrogen bonding (2.22Å ) with nitrogen of triazole ring of synthesized compound 8a. The amino acid residue CYS506 (2.07Å ) had formed hydrogen bonding with oxygen of -NO2 and ILE507 (1.54 and 2.01Å ) had formed hydrogen bonding with oxygen of -NO2 and nitrogen of -NO2 of compound 8b. The triazole ring of compound 8g had shown hydrogen bonding with amino acid residues TYR513 (1.60 and 2.30Å ) and ALA512 (2.33Å ). The binding interactions for compound 8f and fluconazole are shown in Fig. 2. The fluorogroup at para position of benzyl ring is most active compound 8f fitted well into the hydrophobic pocket. On the basis of activity data and docking result,it was found that compound 8f had potential to inhibit cytochrome P450 lanosterol 14α-demethylase of C. albicans.
|
|
Table 2 Molecular docking statistics of synthesized compounds 8a-h. |

|
Download:
|
| Fig. 2.Molecular docking of compound 8f and fluconazole. Ligands are shown in red color. Hydrogen bonds are shown in green color. Hydrophobic bonds are shown in sky blue color. | |
A computational study of all the synthesized compounds was performed for prediction of ADME properties and the value obtained is presented in Table 3. It is observed that compounds exhibited a good % ABS (% absorption) ranging from 59.90% to 86.31%. Furthermore,only compounds 8a and 8b violated Lipinski’s rule of five (miLogP ≤ 5). Remaining all other compounds did not violated Lipinski’s rule of five.
|
|
Table 3 Pharmacokinetic parameters important for good oral bioavailability of the synthesized compounds 6, 7 and 8a-h. |
A molecule likely to be developed as an orally active drug candidate should not show more than one violation of the following four criteria: miLogP (octanol-water partition coefficient) ≤ 5,molecular weight ≤ 500,number of hydrogen bond acceptors ≤ 10 and number of hydrogen bond donors ≤ 5 [39]. All the tested compounds except 8a and 8b followed the criteria for orally active drug and therefore,these compounds may have a good potential for eventual development as oral agents.
4. ConclusionIn summary,we have synthesized new triazole-based coumarin derivatives via click chemistry and evaluated for biological activity. The synthesized compounds show promising antioxidant and antifungal activity when compared with the respective standard drugs. Compound 8a shows potential antioxidant activity (IC50 = 15.20 μg/mL) when compared with standard BHT. Compound 8d,8e and 8f displayed significant antifungal activity when compared with the standard antifungal drug miconazole. In addition to this,molecular docking study of these synthesized triazole derivatives have a high affinity toward the active site of enzyme P450 cytochrome lanosterol 14α-demethylase which provides a strong platform for new structure-based design efforts. Furthermore,analysis of the ADME parameters for synthesized compounds shown good drug-like properties and can be developed as oral drug candidate. Thus,suggesting that compounds from present series 8a (antioxidant activity),8d,8e and 8f (antifungal activity) can be further optimized and developed as a lead molecule.
AcknowledgmentsThe authors M.H.S. and D.D.S. are very much grateful to the Council of Scientific and Industrial Research (CSIR),New Delhi for the award of senior research fellowship. Authors are also thankful to the Head,Department of Chemistry,Dr. Babasaheb Ambedkar Marathwada University,for providing laboratory facility.
| [1] | D.J. Sheehan, C.A. Hitchcock, C.M. Sibley, Current and emerging azole antifungal agents, Clin. Microbiol. Rev. 12 (1999) 40-79. |
| [2] | R. Cha, J.D. Sobel, Fluconazole for the treatment of candidiasis: 15 years experience, Expert Rev. Anti-Infect. Ther. 2 (2004) 357-366. |
| [3] | N.H. Georgopapadakou, T.J. Walsh, Antifungal agents: chemotherapeutic targets and immunologic strategies, Antimicrob. Agents Chemoth. 40 (1996) 279-291. |
| [4] | M.A. Pfaller, S.A. Messer, R.J. Hollis, R.N. Jones, In vitro activities of posaconazole (Sch 56592) compared with those of itraconazole and fluconazole against 3,685 clinical isolates of Candida spp. and Cryptococcus neoformans, Antimicrob. Agents Chemoth. 45 (2001) 2862-2864. |
| [5] | L. Jeu, F.J. Piacenti, A.G. Lyakhovetskiy, H.B. Fung, Voriconazole, Clin. Ther. 25 (2003) 1321-1381. |
| [6] | G.I. Lepesheva, N.G. Zaitseva, W.D. Nes, et al., CYP51 from trypanosomacruzi: a phyla-specific residue in the B0 helix defines substrate preferences of sterol 14ademethylase, J. Biol. Chem. 281 (2006) 3577-3585. |
| [7] | (a) K. Ilango, C.R. Biju, In silico docking investigation, synthesis and cytotoxic studies of coumarin substituted 1, 3, 4-oxadiazole derivatives, J. Pharm. Res. 5 (2012) 1514-1517; |
| [8] | (a) M. Raghu, A. Nagaraj, C.S. Reddy, Synthesis and in vitro study of novel bis-[3-(2-arylmethylidenimino-1,3-thiazol-4-yl)-4-hydroxy-2H-chromen-2-one-6-yl] methane and bis-[3-(2-arylidenhydrazo-1,3-thiazol-4-yl)-4-hydroxy-2Hchromen-2-one-6-yl] methane as potential antimicrobial agents, J. Heterocyclic Chem. 46 (2009) 261-267; |
| [9] | (a) J. Sun, W.X. Ding, K.Y. Zhang, Y. Zou, Efficient synthesis and biological evaluation of 4-arylcoumarin derivatives, Chin. Chem. Lett. 22 (2011) 667-670; |
| [10] | R.M. Patel, N.J. Patel, In vitro antioxidant activity of coumarin compounds by DPPH, super oxide and nitric oxide free radical scavenging methods, J. Adv. Pharm. Technol. Res. 1 (2011) 52-68. |
| [11] | A. Murakami, G.X. Gao, M. Omura, et al., 1,1-Dimethylallylcoumarins potently supress both lipopolysaccharide-and interferon-γ-induced nitric oxide generation in mouse macrophage RAW 264.7 cells, Bioorg. Med. Chem. Lett. 10 (2000) 59-62. |
| [12] | R.G. Lima-Neto, N.N.M. Cavalcante, R.M. Srivastava, et al., Synthesis of 1,2,3-triazole derivatives and in vitro antifungal evaluation on Candida strains, Molecules 17 (2012) 5882-5892. |
| [13] | (a) N. Boechat, V.F. Ferreira, S.B. Ferreira, et al., Novel 1,2,3-triazole derivatives for use against Mycobacterium tuberculosis H37Rv (ATCC 27294) strain, J. Med. Chem. 54 (2011) 5988-5999; |
| [14] | S.G. Agalave, S.R. Maujan, V.S. Pore, Click chemistry: 1,2,3-triazoles as pharmacophores, Chem. Asian J. 6 (2011) 2696-2718. |
| [15] | M.R. Senger, L.D.C.A. Gomes, S.B. Ferreira, et al., Kinetics studies on the inhibition mechanism of pancreatic a-amylase by glycoconjugated 1H-1,2,3-triazoles: a new class of inhibitors with hypoglycemiant activity, Chem. Biol. Chem. 13 (2012) 1584-1593. |
| [16] | X. Zhao, B.W. Lu, J.R. Lu, et al., Design, synthesis and antimicrobial activities of 1,2,3-triazole derivatives, Chin. Chem. Lett. 23 (2012) 933-935. |
| [17] | R.J. Bochis, J.C. Chabala, E. Harris, et al., Benzylated 1,2,3-triazoles as anticoccidiostats, J. Med. Chem. 34 (1991) 2843-2852. |
| [18] | (a) J.L. Kelley, C.S. Koble, R.G. Davis, et al., 1-(Fluorobenzyl)-4-amino-1H-1,2,3-triazolo[4,5-c] pyridines: synthesis and anticonvulsant activity, J. Med. Chem. 38 (1995) 4131-4134; |
| [19] | R. Raj, P. Singh, P. Singh, et al., Azide-alkyne cycloadditionen route to 1H-1,2,3-triazole-tethered 7-chloroquinoline-isatin chimeras: synthesis and antimalarial evaluation, Eur. J. Med. Chem. 62 (2013) 590-596. |
| [20] | A.K. Jordao, P.P. Afonso, V.F. Ferreira, et al., Antiviral evaluation of N-amino-1,2, 3-triazoles against cantagalo virus replication in cell culture, Eur. J. Med. Chem. 44 (2009) 3777-3783. |
| [21] | B.L. Wilkinson, H. Long, E. Sim, A.J. Fairbanks, Synthesis of Arabino glycosyltriazoles as potential inhibitors of mycobacterial cell wall biosynthesis, Bioorg. Med. Chem. Lett. 18 (2008) 6265-6267. |
| [22] | M. Kume, T. Kubota, Y. Kimura, et al., Orally active cephalosporins Ⅱ. Synthesis and structure-activity relationships of new 7-β-[(Z)-2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamido]-cephalosporins with 1,2,3-triazole in C-3 side chain, J. Antibiot. 46 (1993) 177-192. |
| [23] | Y. Shi, C.H. Zhou, Synthesis and evaluation of a class of new coumarintriazole derivatives as potential antimicrobial agents, Bioorg. Med. Chem. Lett. 21 (2011) 956-960. |
| [24] | K. Kushwaha, N. Kaushik, Lata, S.C. Jain, Design and synthesis of novel 2Hchromen-2-one derivatives bearing 1,2,3-triazole moiety as lead antimicrobials, Bioorg. Med. Chem. Lett. 24 (2014) 1795-1801. |
| [25] | R.A. Kusanur, M.V. Kulkarni, New 1,3-dipolar cycloadducts of 3-azidoacetylcoumarins with DMAD and their antimicrobial activity, Indian J. Chem. B 44 (2005) 591-594. |
| [26] | (a) M.H. Shaikh, D.D. Subhedar, L. Nawale, et al., 1,2,3-Triazole derivatives as antitubercular agents; Synthesis, biological evaluation and molecular docking study, Med. Chem. Commun. 6 (2015) 1104-1116; |
| [27] | D. Greenwood, R.C.B. Slack, J.F. Peutherer, Medical Microbiology, 14th ed., ELBS, London, 1992. |
| [28] | M. Burits, F. Bucar, Antioxidant activity of nigella sativa essential oil, Phytother. Res. 14 (2000) 323-328. |
| [29] | T.A. Halgren, Merck molecular force field. I. Basis form, scope, parameterization, and performance of MMFF94, J. Comput. Chem. 17 (1996) 490-519. |
| [30] | R.W.W. Hooft, G. Vriend, C. Sander, E.E. Abola, Errors in protein structures, Nature 381 (1996) 272. |
| [31] | VLife Molecular Design Suite 4.3, VLife Sciences Technologies Pvt. Ltd., www.Vlifesciences. com. |
| [32] | C.A. Lipinski, L. Lombardo, B.W. Dominy, P.J. Feeney, Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings, Adv. Drug Deliv. Rev. 46 (2001) 3-26. |
| [33] | Molinspiration Chemoinformatics Brastislava, Slovak Republic, Available from: http://www.molinspiration.com/cgi-bin/properties 2014. |
| [34] | Y.H. Zhao, M.H. Abraham, J. Le, et al., Rate-limited steps of human oral absorption and QSAR studies, Pharm. Res. 19 (2002) 1446-1457. |
| [35] | Drug-likeness and molecular property prediction, available from: http://www.molsoft.com/mprop/. |
| [36] | S.G. Alvarez, M.T. Alvarez, A practical procedure for the synthesis of alkyl azides at ambient temperature in dimethyl sulfoxide in high purity and yield, Synthesis 4 (1997) 413-414. |
| [37] | W.L. Mendelson, S. Hayden, Preparation of 2,4-dihydroxybenzaldehyde by the Vilsmeier-Haack reaction, Synthetic Commun. 26 (1996) 603-610. |
| [38] | K.K. Srinivasan, Y. Neelima, J. Alex, et al., Synthesis of novel furobenzopyrone derivatives and evaluation of their antimicrobial and antiinflammatory activity, Indian J. Pharm. Sci. 69 (2007) 326-331. |
| [39] | P. Ertl, B. Rohde, P. Selzer, Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties, J. Med. Chem. 43 (2000) 3714-3717. |
 2016, Vol.27
2016, Vol.27