Alphα-glucosidase (EC 3.2.1.20,α-D-glucoside glucohydrolase) plays a key role in the synthesis and processing of di-,oligo-,and polysaccharide chains of α-linked glycoproteins in the endoplasmic reticulum and in the metabolism of polysaccharides and glycoconjugates [1]. Thus,α-glucosidase has been considered as a treatment option for the regulation of postprandial hyperglycemia,which is caused by metabolic disorders that occur in type II diabetes mellitus (noninsulin-dependent diabetes mellitus,NIDDM). WHO projects that diabetes will be the seventh leading cause of death worldwide in 2030; type II diabetes comprises 90% of the diabetes population [2]. High blood glucose concentrations,abnormalities in glycoprotein metabolism caused by insulin resistance,impaired hepatic regulatory effects,and a decrease in glucose utilization are common symptoms in type II diabetes patients [3]. However,the postprandial increase in glucose and plasma insulin levels can be reduced by the inhibition of α-glucosidase activity,which is involved in oligosaccharide hydrolysis and intestinal glucose absorption [4]. By retarding the hydrolysis of the complex carbohydrates,postprandial glucose absorption can be subsided by controlling blood-sugar levels [5]. Alphα-glucosidase inhibitors are promising therapeutic agents,acting as mechanistic probes to reduce the rate of carbohydrate digestion,and will eventually alleviate the levels of postprandial blood glucose and insulin [6]. Thus,to date,α-glucosidase inhibitors have gained considerable attention for their implication in the prevention or treatment of type II diabetes mellitus.
Nitrogen-based heterocyclic organic molecules are an important class of inhibitors used in pharmaceutical chemistry because of the electron-donating characteristics of the nitrogen. The pharmacokinetic and bioavailability properties of the imidazole functional group in the protein histidyl residue derivatives have long fascinated researchers; they provide medicinal activities such as HIV-1 protease inhibitors [7],kinase inhibitors [8],cytochrome enzymes inhibitors [9],potent Jak2 inhibitors [10],phosphodiesterase inhibitors [11],diabetes and obesity drugs [12],and others. The imidazole ring was of interest because it contains two nitrogen atoms,one of which protonates at pH range 6-7 [13],providing more binding sites with a variety of enzymes and proteins,in addition to other electron-donor properties. Gluco-configured imidazoles and cellobiose-derived imidazoles have been synthesized as potential glycosidase inhibitors in the past [14]. The biological characteristics of Schiff bases have been well studied,including their antibacterial,antifungal,and anticancer properties. The presence of the ortho-hydroxyl group in salicylaldehyde,the azomethine (C=N) linkage in the Schiff-base,and the carboxylate group (COO-) in the amino acids provide an electron-rich center to chelate with metal ions. At the same time,the metal ions stabilize the rigid structure of the unstable ligand [15]. Because of their interesting structural features as well as their biological activities,an attempt to research the transition metal complexes derived from amino acids has been reported [16, 17, 18]. Structural studies on the Schiff-base ligand metal complexes derived from various amino acids and salicylaldehyde have been well documented. Moreover,metal complexes of Schiff bases derived from salicylaldehydes and amino acids take part in a variety of biological processes and pharmaceutical fields,for example carboxylation,biological racemization [19],antibacterial and antifungal agents [13],and anticancer drugs [20]. Transition metal-based drugs have existed since the 1960s; for instance,platinum-containing drugs,cisplatin,carboplatin,and oxaliplatin,are known as the most widely used anticancer drugs. New metal complexes have been applied in the therapy and diagnosis of diabetes [21]. However,little effort has been expended to silver-based drug development. Silver(I) ions are known to have antimicrobial properties; normally,other biological activities are ignored. Silver(I) complexes derived from amino acid ligands are considered model complexes used to gain insight into the silver(I)-protein interactions [22].
In summary,silver(I) complexes of salicylaldehyde derived from histidine Schiff bases are worth researching as a novel class of α-glucosidase inhibitors; they demonstrate their potential usage in treating PPHG (postprandial hyperglycemia),which can provide a promising avenue for controlling or regulating α-glucosidase activity in order to treat or prevent diseases caused by metabolic disorders such as type II diabetes mellitus. In this study,in order to generate a novel,easily accessible,and high-affinity α-glucosidase inhibitor,we investigated the inhibition properties of complexes with the Schiff bases derived from salicylaldehyde for the first time and explored the inhibition mechanism further.
2. Experimental 2.1. Synthesis of silver(I) complexes (1a-9a)The preparations of the histidine Schiff bases were carried out in the following general solid-state grinding procedure: 1 mmol amino acid,aldehydes and KOH were ground until finely blended in an agate mortar. Deprotonation of ligands becomes easier for the addition of KOH. The reactants were then placed in a microwave oven (500-800 W) for 5 min (grind 15 s min-1). The reaction products were obtained,recrystallized,filtered under reduced pressure,washed with ethanol,and finally dried by the infrared light,with 70%-90% yield. All the amino acid Schiff bases are coloured solids and air stable for an extended period of time. They are soluble in water and DMSO at room temperature. All of them had melting points above 280 ℃.
The newly synthesized Schiff base (0.1mmol) and silver acetate (0.1 mmol) were mixed also in an agate mortar. 2-3 drops of ethyl alcohol were added during the grinding. The reactants were then placed in a microwave oven (800 W) for 3 min (grind 30 s min-1 ). The target complexes were obtained,recrystallized,filtered under reduced pressure,washed with 30%-50% aqueous ethanolic solution and finally dried over by the infrared light,with 70%- 90% yield. The complexes are insoluble in water,sparingly soluble in ethyl alcohol and DMSO at room temperature,with solubility increasing with temperature. These compounds are also stable for a fairly long time at room temperature in the solid state. All of them the melting point are above 280 ℃.
Referring to the coordination mode of azo-linked Schiff base Cu(II) [23],we hypothesized the common structure of complexes is 1a-9a. Terdentate complexes were obtained upon reaction between metal ions and ligands at 1:1 molar ratio. The determined structural information needed further confirmation.
2.2. Bioassay procedures2.2.1. Assay for α-glucosidase inhibitory activity [24] and [25]
The enzyme and the substrate solution was prepared by dissolving Saccharomyces cerevisiae α-glucosidase in 0.01 mol L-1 potassium phosphate buffer (pH 7). Diluted enzyme solution (10 μL),test samples (0.016-2 μL,in DMSO) and buffer solution (90 μL) were mixed in each well of a 96-well microtiter plate. After pre-incubating for 20 min at 37 ℃,PNPG (10 μL,1.5 mg mL-1) was added to start the enzymatic reaction measured by a microtiter plate reader immediately. The increment of absorption at 405 nm is based on the hydrolysis of PNPG. Controls without enzyme or without substrate were included. The resveratrol was used as reference and averages of three replicates were presented. The inhibition percentage (%) was calculated by the equation: [Abssample/Absblank] × 100(%),where Absblank represents the absorbance of the blank with the same volume DMSO.
2.2.2. Kinetic assayThe enzyme solution (10 μL),0.01 mol L-1 potassium phosphate buffer,and test samples (2 μL,in DMSO) were mixed in a 96- well microtiter plate. After incubation at 37 ℃ for 20 min,PNPG (0.45-1.50 mg μL-1) was added and measured by a microtiter plate reader at 405 nm straight away. The X-axis of Lineweaver- Burk plot is the reciprocal of the concentration of PNPG; the Y-axis is the reciprocal of the rate of enzyme reaction. All the experiments were carried out in triplicate.
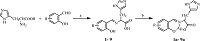
3. Results and discussion 3.1. Inhibitory activity of α-glucosidaseIn this paper,we disclose a simple,new synthesis of histidine Schiff base complexes; in the absence of an added solvent,two macroscopic solids interact directly (Scheme 1). The condensation of amines and aldehydes to azomethines in the solid state was recently reported to produce high yields at elevated temperatures. Grinding the solid aldehydes and amino acids together without the addition of a catalyst requires a liquid phase before the completion of the reaction,where a liquid melt would be observed [26].
|
Download:
|
| Scheme 1.Synthetic route for compounds 1-9 and 1a-9a. Reagents and condition: (a) 1 mmol histidine amino acid, aldehydes and KOH were grinding until finely blended in an agate mortar. Placed in a microwave oven (500-800 W) for 5 min (grind 15 s min-1). Recrystallized with ethanol. Soluble in water and DMSO at room temperature. (b) The newly synthesized Schiff base (1 mmol) and silver acetate (1 mmol) were mixed also in an agate mortar. 2-3 drops of ethyl alcohol were added. Placed in a microwave oven (800 W) for 3 min (grind 30 s min-1 [2TD$DIF]). Recrystallized with 30-50% aqueous ethanolic solution. | |
In order to delineate the structure-activity relationship,and to get an optimized α-glucosidase inhibitor,salicylaldehyde was substituted with electron donating groups such as hydroxylwithdrawing groups; electron withdrawing groups such as chloro and bromine groups were used in the synthesis of the histidine Schiff base complexes. Different numbers and positions of the substituents were also taken into consideration.
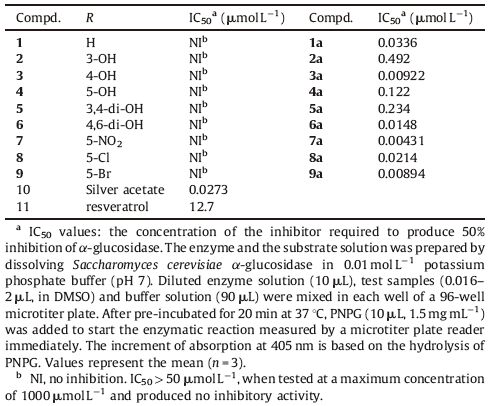
The structure-activity relationships of the compounds are shown in Table 1; all of the silver complexes demonstrated high activity with IC50 values ranging from 0.00431 mmol L-1 to 0.492 mmol L-1. Compound 5-nitrosalicylaldehyde Schiff base silver complex 7a,bearing a nitro group at the 5-position of the salicylaldehyde,was proved to be the most active with an IC50 value of 0.00431 mmol L-1 ,showing 3000 times higher activity than the reference inhibitor 11. However,most of the histidine amino acid Schiff bases 1-9 showed little inhibition. The inhibition screening results exhibited marked enhancement in activity with coordination of the silver ions against the α-glucosidase. This enhancement in activity can be rationalized on the basis of the structures of the ligands because it processes an additional azomethine (C=N) linkage that is important in the coordination of the silver ion. The polarity of the metal ion was reduced by chelation,creating a positive charge that was shared with the donor groups of ligands and delocalized π-electrons over the entire chelating ring [27]. However,the reactant silver acetate 10 displayed inhibitory activities with IC50 values of 0.0273 mmol L-1. Not all of the complexes possessed better inhibitory activities compared to the central metal,silver(I). The results revealed that the inhibition of the Schiff base metal complexes was decided not only on the silver ions,but also on the ligand. The benzene ring of the salicylaldehyde and the imidazole ring serve to improve binding and to characterize enzymes possessing multiple subsites. The silver ion is the key feature of these compounds contributing to their binding efficiency,while the imidazole ring provides noncovalent and electrostatic interactions,features a diploid occupied nitrogen orbital for antiprotonation,and is thoroughly electronegative enough to permit a simultaneous interaction with the catalytic nucleophile [28].
|
|
Table 1 Reaction and the IC50 values of silver complexes of histidine schiff base and the reference inhibitors. |
By comparing two compounds at a time,1a,2a,3a and 4a,2a and 5a,it is obvious that the R2 = 4-OH substituent played an important role in promoting enzymatic activity. Changing the position of the hydroxyl group in the phenyl ring,from the 4- position to another position,produced diminished activity. The improper positions of hydroxyl groups had a negative impact on the inhibitory activity,even if the number of hydroxyl groups increased. When compounds possessed the R2 = 3-OH substituent,such as 2a and 5a,they exhibited lower activity than other compounds in the same series; this indicates that the R2 = 3-OH substituent may be unfavorable for binding with α-glucosidase. It was also observed that the modification of bromine 9a with chlorine 8a reduced the inhibitory activity. From the activity pattern,it can be generalized that the presence of electronwithdrawing groups such as nitro,chloro,and bromine groups on the phenyl ring play a more important role in promoting activity than the electron-donating groups. It was deduced that hydrophobic amino acid residues in the active sites of α-glucosidase are thought to be involved in the hydrophobic interaction of the halogen atom on the benzene ring.
In addition,several metal complexes of 2,4-dihydroxybenzaldehyde- histidine Schiff base,including Bi3+,Ce3+,Ni2+,Sn4+,Al3+,Zn2+,Mn2+,and Fe2+ were synthesized and screened for inhibitory activities in vitro which displayed IC50 values greater than 50 mmol L-1.
3.2. Kinetics and the mechanism of α-glucosidase inhibitionEnzyme kinetics can provide valuable information to clarify how and where the ligand complexes bind to α-glucosidase. The obtained Lineweaver-Burk plots are shown in Fig. 1. The plots illustrate that compounds 7a and 9a are typical noncompetitive aglucosidase inhibitors. Both compounds 7a and 9a bound to the noncompetitive domain of S. cerevisiae α-glucosidase rather than to the active site and were not influenced by substrate concentrations. Referring to the interaction patterns studied by Dimova et al. [8],a critically important interaction was formed between the backbone N,NH of the imidazole,and the Ser244 or Arg212 residue,which is near the active site of the S. cerevisiae aglucosidase [29]. The imidazole ring created a π-cation in a hydrogen bond with Ser244,the imidazole nitrogen accepted a proton from the enzymatic acid,and its NH fragment was involved in a hydrogen bond with an amino group. The 4-bromobenzene ring may be accommodated in the hydrophobic region. Moreover,the silver atoms re-coordinated with other amino acid residues to achieve better integration with the enzyme. The exact results require further confirmation by additional experiments. Yet,the docking studies carried by Yar et al. [30] have confirmed that the NH fragment of the imidazole and the hydroxyl group connected to the aromatic ring were responsible for making hydrogen bonds,thus making the molecule more promising.

|
Download:
|
| Fig. 1.Double-reciprocal plots of the inhibiton kinetics of Saccharomyces cerevisiae α-glucosidase by compounds (A) 7a and (B) 9a. α-Glucosidase and compounds were mixed and incubated at 37 8C for 20 min, and the PNPG of varying concentrations was added to initiate the enzymatic reaction. | |
In summary,these findings clearly indicate that transition metal based complexes and imidazoles have the potential for practical applications such as the development of new therapeutic reagents for diseases. Evaluation of the α-glucosidase inhibitory activity of these silver complexes revealed that all of them presented excellent α-glucosidase inhibition with IC50 values at low micro-molar concentrations ranging from 0.00431 mmol L-1 to 0.492 mmol L-1. Among the synthesized compounds,compound 7a,5-nitro salicylaldehyde Schiff base silver complex,displayed the highest inhibitory activity with an IC50 value of 0.00431 mmol L-1. From the drug design standpoint,combined administrations of metal ions may serve as a promising strategy to enhance inhibitory activity. The inhibition kinetics study indicated that this class of silver(I) complexes uses a noncompetitive mechanism. Hence,due to their extraordinary inhibitory activity,they may provide a new chemical class for type II diabetes therapy.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2015.11.015.
| [1] | N. Asano, Glycosidase inhibitors: update and perspectives on practical use, Glycobiology 13 (2003) 93R-104R. |
| [2] | World Health Organization, Diabetes, Available at (http://www.who.int/mediacentre/factsheets/fs312/en/index.html). |
| [3] | O. Vahidi, K.E. Kwok, R.B. Gopaluni, L. Sun, Developing a physiological model for type Ⅱ diabetes mellitus, Biochem. Eng. J. 55 (2011) 7-16. |
| [4] | A. Trapero, A. Llebaria, A prospect for pyrrolidine iminosugars as antidiabetic aglucosidase inhibitors, J. Med. Chem. 55 (2012) 10345-10346. |
| [5] | H.W. Ryu, B.W. Lee, M.J. Curtis-Long, et al., Polyphenols from Broussonetia papyrifera displaying potent α-glucosidase inhibition, J. Agric. Food Chem. 58 (2010) 202-208. |
| [6] | Y. Liu, L. Ma, W.H. Chem, et al., Binding mechanism and synergetic effects of xanthone derivatives as noncompetitive α-glucosidase inhibitors: a theoretical and experimental study, J. Phys. Chem. B 117 (2013) 13464-13471. |
| [7] | S.S.Abdel-Meguid,B.W.Metcalf, T.J. Carr, et al.,Anorally bioavailableHIV-1 protease inhibitor containing an imidazole-derived peptide bond replacement: crystallographic and pharmacokinetic analysis, Biochemistry 33 (1994) 11672-11677. |
| [8] | D. Dimova, P. Iyer, M. Vogt, et al., Assessing the target differentiation potential of imidazole-based protein kinase inhibitors, J. Med. Chem. 55 (2012) 11067-11071. |
| [9] | A. Verras, I.D. Kuntz, P.R. Ortiz de Montellano, Computer-assisted design of selective imidazole inhibitors for cytochrome P450 enzymes, J. Med. Chem. 47 (2004) 3572-3579. |
| [10] | Q.B. Su, S. Ioannidis, C. Chuaqui, et al., Discovery of 1-methyl-1H-imidazole derivatives as potent Jak2 inhibitiors, J. Med. Chem. 57 (2014) 144-158. |
| [11] | R. Buchman, P.F. Heinstein, J.N. Wells, Imidazole derivatives as inhibitors of cyclic nucleotide phosphodiesterases, J. Med. Chem. 17 (1974) 1168-1173. |
| [12] | S.W. He, Q.M. Hong, Z. Lai, et al., Discovery of a potent and selective DGAT1 inhibitor with a piperidinyl-oxy-cyclohexanecarboxylic acid moiety, Med. Chem. Lett. 5 (2014) 1082-1087. |
| [13] | L.H. Abdel-Rahman, R.M. EI-Khatib, L.A.E. Nassr, A.M. Abu-Dief, F.E.D. Lashin, Design, characterization, teratogenicity testing, antibacterial, antifungal and DNA interaction of few high spin Fe(Ⅱ) Schiff base amino acid complexes, Spectrochim. Acta A 111 (2013) 266-276. |
| [14] | A. Varrot, M. Schülein, M. Pipelier, A. Vasella, G.J. Davies, Lateral protonation of a glycosidase inhibitor. Structure of the Bacillus agaradhaerens Cel5A in complex with a cellobiose-derived imidazole at 0. 97A˚ resolution, J. Am. Chem. Soc. 121 (1991) 2621-2622. |
| [15] | A. Trzesowska-Kruszynska, Copper complex of glycine schiff base: in situ ligand synthesis, structure, spectral, and thermal properties, J. Mol. Struct. 1017 (2012) 72-78. |
| [16] | N. Raman, A. Sakthivel, N. Pravin, Exploring DNA binding and nucleolytic activity of few 4-aminoantipyrine based amino acid schiff base complexes: a comparative approach, Spectrochim. Acta A 125 (2014) 404-413. |
| [17] | R. Ando, H. Inden, H. Sugino, et al., Spectroscopic characterization of amino acid and amino acid ester-Schiff-base complexes of oxovanadium and their catalysis in sulfide oxidation, Inorg. Chim. Acta 357 (2004) 1337-1344. |
| [18] | R. Ganguly, B. Sreenivasulu, J.J. Vittal, Amino acid-containing reduced schiff bases as the building blocks for metallasupramolecular structures, Coordin. Chem. Rev. 252 (2008) 1027-1050. |
| [19] | A. Pasini, L. Casella, Some aspects of the reactivity of amino acids coordinated to metal ions, J. Inorg. Nucl. Chem. 36 (1974) 2133-2144. |
| [20] | J. Zuo, C.F. Bi, Y.H. Fan, et al., Cellular and computational studies of proteasome inhibition and apoptosis induction in human cancer cells by amino acid schiff base-copper complexes, J. Inorg. Biochem. 118 (2013) 83-93. |
| [21] | L. Ronconi, P.J. Sadler, Using coordination chemistry to design new medicines, Coor. Chem. Rev. 251 (2007) 1633-1648. |
| [22] | N.C. Kasuga, M. Sato, A. Amano, et al., Light-stable and antimicrobial active silver(I) complexes composed of triphenylphosphine and amino acid ligands: Synthesis, crystal structure, and antimicrobial activity of silver(I) complexes constructed with hard and soft donor atoms (n∞{[Ag(L)(PPh3)] 2} with L = α-ala- or asn- and n = 1 or 2), Inorg. Chim. Acta 361 (2008) 1267-1273. |
| [23] | S.R. Moamen, I.M. El-Deen, K.I. Hassan, S. El-Ghool, Synthesis and spectroscopic studies of some transition metal complexes of a novel Schiff base ligands derived from 5-phenylazo-salicyladehyde and o-amino benzoic acid, Spectrochimi. Acta. A 65 (2006) 1208-1220. |
| [24] | G. Pistia-Brueggeman, R.I. Hollingsworth, A preparation and screening strategy for glycosidase inhibitors, Tetrahedron 57 (2001) 8773-8778. |
| [25] | J.S. Kim, Y.S. Kwon, Y.J. Sa, M.J. Kim, Isolation and identification of sea buckthorn (Hippophae rhamnoides) phenolics with antioxidant activity and α-glucosidase inhibitory effect, J. Agric. Food Chem. 59 (2011) 138-144. |
| [26] | G. Rothenberg, A.P. Downie, C.L. Raston, J.L. Scott, Understanding solid/solid organic reactions, J. Am. Chem. Soc. 123 (2001) 8701-8708. |
| [27] | B.K. Singh, H.K. Rajour, A. Prakash, Synthesis, characterization and biological activity of transition metal complexes with Schiff bases derived from 2-nitrobenzaldehyde with glycine and methionine, Spectrochim. Acta A 94 (2012) 143-151. |
| [28] | T.D. Heightman, A. Vasella, K.E. Tsitsanou, et al., Cooperative interactions of the catalytic nucleophile and the catalytic acid in the inhibition of β-glycosidases. Calculations and their validation by comparative kinetic and structural studies of the inhibition of glycogen phosphorylase b, Helv. Chim. Acta 81 (1998) 853-864. |
| [29] | K. Bharatham, N. Bharatham, K.H. Park, K.W. Lee, Binding mode analyses and pharmacophore model development for sulfonamide chalcone derivatives, a new class of α-glucosidase inhibitors, J. Mol. Graphics Modell. 26 (2008) 1202-1212. |
| [30] | N. Yar, M. Bajda, S. Shahzad, et al., Organocatalyzed solvent free an efficient novel synthesis of 2,4,5-trisubstituted imidazoles for α-glucosidase inhibition to treat diabetes, Bioorg. Chem. 58 (2015) 65-71. |
 2016, Vol.27
2016, Vol.27