b University of Chinese Academy of Sciences, Beijing 100039, China
The development of efficient methodologies to provide optically active products has aroused great interest between academia and industry due to the ever increasing demands for chiral chemicals. Among the various approaches employed for this purpose,asymmetric catalysis represents one of the most general and attractive strategies in terms of chirality economy and environment considerations [1, 2]. The asymmetric conjugate addition (ACA) of carbon nucleophiles to α,β-unsaturated compounds is an important method for carbon-carbon bond formation in asymmetric catalysis [3, 4, 5, 6]. To achieve maximum chiral multiplication,an impressive array of chiral ligands,such as phosphoramidite [7, 8, 9, 10, 11, 12, 13, 14],phosphite ligands [15, 16, 17, 18, 19, 20, 21],P,N-ligands [22, 23, 24, 25, 26] and others [27, 28, 29, 30, 31, 32, 33, 34, 35],have been developed to control the stereochemistry of ACA. Among these ligands,phosphite ligands have shown significant promise because of their facile synthetic method,and efficiency for 1,4-addition. In spite of huge achievements in this area,however,further research is needed to understand how to obtain an efficient enantiocontrol [36, 37]. In this context,the design of new ligands is still an important area of research,thus the careful selection of a suitable alcohol backbone has become a meaningful procedure for us.
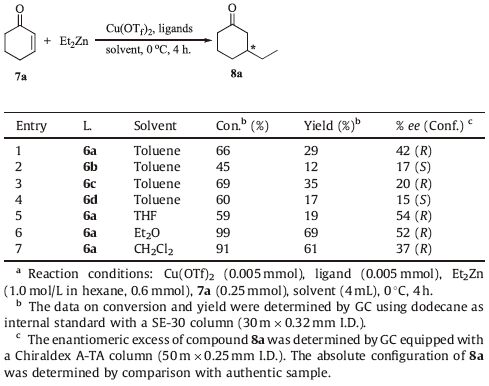
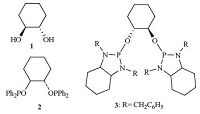
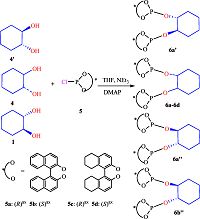
1,2-Cyclohexanediol (CHD) is widely used in preparing polyester,epoxy resin thinner,o-dihydroxybenzene and so on. As an important organic intermediate,CHD has enjoyed great success over the years in the fields of medicine,pesticides,spices and organic synthesis [38, 39, 40, 41]. For example,Spilling et al. [42] found that catalysts formed by mixing (1S,2S)-trans-1,2-cyclohexanediol 1 and Ti(Oi Pr)4 at a 1.1:1 ratio proved to be effective for the phosphonylation of cinnamaldehyde providing hydroxyphosphonate with good enantiomeric excesses (up to 70% ee) (Fig. 1.). Subsequently,RajanBabu et al. [43] undertook a study of the hydrocyanation of 1,3-dienes using bis-1,2-diphenylphosphinite 2 derived from racemic trans-1,2-cyclohexanediol,and over 95% yield was gained. Recently,the Merce` Rocamora group [44] used N,N’-dibenzylcyclohexane-1,2-diamine and CHD as starting materials,and prepared enantiopure bidentate bis(diamidophosphite) ligand 3,which is employed in Rh-catalyzed asymmetric hydrogenation of methyl (Z)-α-acetamidocinnamate with up to 76% ee. Previous results found the CHD skeleton was successfully applied in asymmetric catalytic reactions,and extremely useful for the synthesis of chiral ligands. Based on these findings and considering the importance of the electron density at the phosphorus atom and the configuration of the biaryl moieties in inducing high enantioselectivity,a series of new chiral aryl diphosphite ligands 6a through 6d using racemic trans-1,2- cyclohexanediol as the diol skeleton were designed and synthesized. At the same time,ligands 6a' ,6a'' and 6b'' derived from enantiopure trans-1,2-cyclohexanediol were also prepared to compare to the asymmetric inducing ability of ligands 6a through 6d. The results indicated that ligand 6a gave high activity (97% yield) and enantioselectivity (97% ee) in the Cu-catalyzed ACA of ZnEt2 to 2-cyclohexenone. To our delight,similar results were obtained (98% yield,99% ee) when ligand 6a' was used.
|
Download:
|
| Fig. 1.The representative examples of the application of trans-1,2-cyclohexanediol in organic synthesis. | |
The NMR spectra were recorded on a Bruker 300 MHz,or Bruker 400 MHz spectrometer. The 1H and 13C NMR spectra were reported in parts per million (ppm) with TMS (δ = 0.00 ppm) as an internal standard. The 31P NMR spectra were reported in ppm with 85% H3PO4 as an external reference. Proton chemical shifts (δ) and coupling constants (J) were reported in ppm and Hz,respectively. Spin multiplicities were given as s (singlet),d (doublet),t (triplet) and m (multiplet). High resolution mass spectra (HRMS) were recorded on a Bruker microTOF-QII mass spectrometer. All the melting points were determined on an X-4 melting point apparatus and are uncorrected. Optical rotations were measured on a Perkin- Elmer 241 MC polarimeter at 20 ℃.
All non-aqueous reactions and manipulations were performed under an N2 atmosphere with standard Schlenk techniques. Reactions were monitored by thin layer chromatography (TLC,silica gel GF254 plates). Column chromatography separations were conducted on silica gel (200-300 mesh). Reagents Et3N,THF,Et2O and toluene were distilled with Na and benzophenone as an indicator,and CH2Cl2 was dried over CaH2 before use. The H8- binaphthol was prepared according to a literature procedure [45]. All the other chemicals were obtained commercially and used without further purification.
2.1. Synthesis of diphosphites 6a-6d,6a',6a'' and 6b''As shown in Scheme 1,diphosphite ligands 6a through 6d,6a,6a'' and 6b'' were easily synthesized in one step from racemic trans-1,2-cyclohexanediol 4,(1R,2R)-trans-1,2-cyclohexanediol 40,(1S,2S)-trans-1,2-cyclohexanediol 1,and chlorophosphoric acid diary ester 5,which derived from 2,2’-dihydroxy-1,1’-binaphthol(- binaphthol),and 2,2’-dihydroxy-5,5’,6,6’,7,7’,8,8’-octahydro-1,1’- binaphthol (H8-binaphthol). Ligand 6a through 6d,6a',6a'' and 6b'' were purified on a silica gel column under a nitrogen atmosphere with low to general yields. The 31P NMR,1H NMR and 13C NMR were consistent with the expectation for these ligands. The ratios of the two diastereoisomers for ligands 6a through 6d obtained by the integrated area of two singlets in the 31P NMR of ligands were 1.18,1.00,1.43 and 1.12,respectively. It is worth mentioning that the ratio was changed slightly each time when the same ligand was synthesized.
|
Download:
|
| Scheme 1.The synthesis of diphosphite ligands derived from racemic trans-1,2- cyclohexanediol, (1R,2R)-trans-1,2-cyclohexanediol, and (1S,2S)-trans-1,2-cyclohexanediol. | |
To a 100 mL Schlenk flask equipped with a condenser were added 2.0 g of (R)-binaphthol,20 mL of toluene,and 12 mL of PCl3. Under a nitrogen atmosphere the mixture was refluxed for 20 h. After removal of the excessive PCl3 and toluene,the residue was dissolved in 20 mL of toluene,and then was transferred to another Schlenk flask,and toluene was removed in vacuo to obtain compound (R)-1,1'-binaphthyl-2,20-diyl-chlorophosphite (5a) as a white powder,which was used directly in the following step without further purification. To a stirred solution of compound 4 (87.5 mg,0.75 mmol),compound 5a (529.3 mg,1.51 mmol),and 4- dimethylaminopyridine (DMAP) (18.4 mg,0.15 mmol) in THF (10 mL) at -15 ℃,Et3N (0.32 mL) was slowly added using a syringe over 1 min,and the solution was stirred at -15 ℃ for 0.5 h. The mixture was then stirred at r.t. for 1 h. THF was distilled off in vacuo,and then toluene (20 mL) was added. The solid was removed by filtration through a pad of silica gel,and the solvent was removed under reduced pressure. The residue was purified by flash chromatography (Rf = 0.53, n-hexane:THF = 3:1,v:v),and furnished ligand 6a as a white foamy solid (169.3 mg,30.34% yield). [α]D 20 -253(c 0.19,CH2Cl2); Mp 153-154 ℃; 1H NMR (400 MHz,DMSO-d6): δ 8.16 (dd,2H,J = 8.8,6.4 Hz,Ar),8.10-7.98 (m,6H,Ar),7.58 (d,1H,J = 8.8 Hz,Ar),7.56-7.45 (m,7H,Ar),7.34 (dd,4H,J = 12.4,7.4 Hz,Ar),7.27 (d,1H,J = 8.8 Hz,Ar),7.21 (dd,3H,J = 8.4,2.8 Hz,Ar),4.35-4.13 (m,2H,CH),2.24-2.09 (m,1H,CH2),1.91 (d,1H,J = 12.8 Hz,CH2),1.63 (s,1H,CH2),1.57-1.41 (m,3H,CH2),1.27 (m,2H,CH2). 13C NMR (101 MHz,DMSO-d6): δ 148.10,148.06,147.94,147.91,147.34,147.31,132.45,132.16,131.57,131.28,131.22,131.08,130.46,130.36,129.06,
128.93,127.12,127.11,126.98,126.39,126.34,125.71,125.51,124.07,123.96,123.86,123.81,122.45,122.35,122.32,
122.11,122.02,121.95,77.59,77.38,77.12,76.95,32.76,31.96,23.30,22.62. 31P NMR (162 MHz,DMSO-d6): δ 150.75,149.31. HRMS (ESI+): calcd. for C46H34NaO6P2 [M + Na]+ 767.1723; found: 767.1738.
' Treatment of compound 4' (77.6 mg,0.67 mmol),5a (507.5 mg,1.45 mmol),and DMAP (17.7 mg,0.15 mmol) as described for the synthesis of ligand 6a afforded ligand 6a' ,which was purified by flash chromatography (Rf = 0.48,n-hexane: THF = 3:1) to produce a white solid (223.2 mg,44.77% yield). [α]D 20 -449 (c 0.15,CH2Cl2); Mp 132-133 ℃; 1H NMR (400 MHz,DMSO-d6): δ 8.14 (d,2H,J = 8.8Hz,Ar),8.06 (d,4H,J = 8.2Hz,Ar),7.99 (d,2H,J = 8.8 Hz,Ar),7.47-7.57 (m,6H,Ar),7.44 (d,2H,J = 8.8 Hz,Ar),7.35 (dd,4H,J = 15.6,8.0 Hz,Ar),7.25- 7.29 (m,2H,Ar),7.22 (s,2H,Ar),4.17 (m,2H,CH),2.14 (t,2H,J = 12.0 Hz,CH2),1.62 (s,2H,CH2),1.47 (d,2H,J = 10.0Hz,CH2),1.17-1.27 (m,2H,CH2). 13C NMR (101 MHz,DMSO-d6): δ 147.94,147.34,132.46,132.17,131.58,131.28,131.09,130.36,129.08,128.93,127.13,126.98,126.39,126.34,125.72,
125.68,124.02,123.97,122.45,121.95,77.56,77.39,32.75,23.30. 31P NMR (162 MHz,DMSO-d6): δ 150.74. HRMS (ESI+): calcd. for C46H34NaO6P2 [M + Na]+ 767.1723; found: 767.1725.
2.1.3. (1S,2S)-Bis[(R)-1,10-binaphthyl-2,20-diyl]phosphitecyclohexanediol 6a''
Treatment of compound 4' (77.6 mg,0.67 mmol),5a (507.5 mg,1.45 mmol),and DMAP (17.7 mg,0.15 mmol) as described for the synthesis of ligand 6a afforded ligand 6a'' ,which was purified by flash chromatography (Rf = 0.48,n-hexane:THF = 3:1) to produce a white solid (184.0 mg,36.90% yield). [α]D 20 -246 (c 0.11,CH2Cl2); Mp 112-113 ℃; 1H NMR (400 MHz,DMSO-d6): δ 8.17 (d,2H,J = 8.8 Hz,Ar),8.09 (t,2H,J = 6.8 Hz,Ar),8.03 (t,4H,J = 8.2 Hz,Ar),7.61 (d,2H,J = 8.8 Hz,Ar),7.51 (q,6H,J = 6.2 Hz,Ar),7.35 (t,4H,J = 8.0 Hz,Ar),7.30-7.23 (m,4H,Ar),4.31 (m,2H,CH),1.92 (d,2H,J = 11.8 Hz,CH2),1.79-1.74 (m,1H,CH2),1.60-1.39 (m,5H,CH2). 13C NMR (101 MHz,DMSO-d6): δ 148.12,147.32,132.46,132.17,131.58,131.24,131.10,130.48,129.08,128.96,127.12,127.00,126.42,126.36,125.54,
123.88,123.83,122.33,122.13,122.04,77.17,77.01,31.99,22.65. 31P NMR (162 MHz,DMSO-d6): δ 149.44. HRMS (ESI+): calcd. for C46H34NaO6P2 [M + Na]+ 767.1723; found: 767.1738.
(S)-1,10-Binaphthyl-2,20-diyl-chlorophosphite 5b was synthesized by the same procedure as 5a,and was used directly without further purification. Treatment of compound 4 (62.3 mg,0.54 mmol),5b (412.7 mg,1.18 mmol),and DMAP (14.4 mg,0.12 mmol) as described for the synthesis of ligand 6a afforded ligand 6b,which was purified by flash chromatography (Rf = 0.51,n-hexane:THF = 3:1) to produce a white solid (160.1 mg,39.83% yield). [α]D 20 180 (c 0.18,CH2Cl2); Mp 138-139 ℃; 1H NMR (400 MHz,DMSO-d6): δ 8.16 (dd,2H,J = 8.8,6.4 Hz,Ar),8.05 (dt,6H,J = 22.0,7.6 Hz,Ar),7.59 (d,1H,J = 8.8 Hz,Ar),7.57-7.42 (m,7H,Ar),7.36 (m,4H,Ar),7.30-7.21 (m,4H,Ar),4.24 (m,2H,CH),2.15 (t,1H,J = 12.6 Hz,CH2),1.91 (d,1H,J = 11.6 Hz,CH2),1.63 (s,1H,CH2),1.49 (d,3H,J = 21.6 Hz,CH2),1.25 (d,2H,J = 12.0 Hz,CH2). 13C NMR (101 MHz,DMSO-d6): δ 148.11,148.06,147.93,147.92,147.34,147.31,132.45,132.16,131.57,131.27,131.22,131.08,130.46,130.36,129.06,
128.93,127.12,127.10,126.97,126.40,126.34,125.68,124.02,123.97,123.87,123.82,122.45,122.34,122.32,122.11,
122.02,121.95,77.56,77.39,77.15,76.98,32.77,31.98,23.30,22.63. 31P NMR (162 MHz,DMSO-d6): δ 150.77,149.32. HRMS (ESI+): calcd. for C46H34NaO6P2 [M + Na]+ 767.1723; found: 767.1732.
Treatment of compound 1 (62.3 mg,0.54 mmol),5b (412.7 mg,1.18 mmol),and DMAP (14.4 mg,0.12 mmol) as described for the synthesis of ligand 6a afforded ligand 6b'',which was purified by flash chromatography (Rf = 0.50,n-hexane:THF = 3:1) to produce a white solid (175.1 mg,39.13% yield). [α]D 20 98 (c 0.14,CH2Cl2); Mp 135-136 ℃; 1HNMR (400 MHz,DMSO-d6):δ 8.14 (d,2H,J = 8.8 Hz,Ar),8.10-8.03 (m,4H,Ar),8.00 (d,2H,J = 8.8 Hz,Ar),7.57-7.47 (m,6H,Ar),7.44 (d,2H,J = 8.8 Hz,Ar),7.36 (dd,4H,J = 15.6,7.6 Hz,Ar),7.26 (d,2H,J = 8.4 Hz,Ar),7.23-7.17 (m,2H,Ar),4.18 (m,2H,CH),2.23-2.11 (m,2H,CH2),1.63 (s,2H,CH2),1.48 (d,2H,J = 10.0 Hz,CH2),1.23 (d,2H,J = 7.2 Hz,CH2). 13C NMR (101 MHz,DMSO-d6): δ 147.94,147.33,132.45,132.17,131.57,131.29,131.09,130.37,129.08,128.93,127.14,127.00,126.38,126.34,125.73,
125.52,124.00,123.96,122.45,122.34,77.57,77.39,32.76,23.30. 31P NMR (162 MHz,DMSO-d6): δ 150.72. HRMS (ESI+): calcd. for C46H34NaO6P2 [M + Na]+ 767.1723; found: 767.1733.
(R)-1,1'-H8-Binaphthyl-2,2'-diyl-chlorophosphite 5c was synthesized by the same procedure as 5a,and was used directly without further purification. Treatment of compound 4 (77.9 mg,0.67 mmol),5c (530.0 mg,1.48 mmol),and DMAP (18.0 mg,0.15 mmol) as described for the synthesis of ligand 6a afforded ligand 6c,which was purified by flash chromatography (Rf = 0.54,n-hexane:toluene = 2:1) to produce a white solid (124.0 mg,24.34% yield). [α]D 20 -153 (c 0.11,CH2Cl2); Mp 91-92 ℃; 1H NMR (400 MHz,DMSO-d6): δ 7.13 (d,2H,J = 8.8 Hz,Ar),7.04 (dd,3H,J = 15.2,8.0 Hz,Ar),6.98 (d,1H,J = 8.0 Hz,Ar),6.86 (dd,2H,J = 16.0,8.2 Hz,Ar),4.09 (m,2H,CH),2.90-2.54 (m,12H,CH2),2.23-2.02 (m,6H,CH2),1.86-1.59 (m,14H,CH2),1.58-1.32 (m,8H,CH2). 13C NMR (101 MHz,DMSO-d6): δ 146.32,146.17,145.83,145.80,138.37,138.26,137.35,137.29,134.99,134.90,133.89,133.81,129.84,129.82,129.40,
129.39,127.75,127.61,127.60,119.37,119.23,119.06,118.99,77.18,77.15,76.97,76.95,32.76,28.83,27.70,27.67,27.58,
23.39,22.49,22.40,22.38,22.32,22.27. 31P NMR (162 MHz,DMSO-d6): δ 145.11,142.80. HRMS (ESI+): calcd. for C46H50NaO6P2 [M + Na]+ 783.2975; found: 767.3010.
(S)-1,1'-H8-Binaphthyl-2,2'-diyl-chlorophosphite 5d was synthesized by the same procedure as 5a,and used directly without further purification. Treatment of compound 4 (64.9 mg,0.56 mmol),5d (500.0 mg,1.40 mmol),and DMAP (17.1 mg,0.14 mmol) as described for the synthesis of ligand 6a afforded ligand 6d,which was purified by flash chromatography (Rf = 0.45,n-hexane:toluene = 2:1) to produce a white solid (132.7 mg,31.16% yield). [α]D 20 170 (c 0.10,CH2Cl2); Mp 105-106 ℃; 1H NMR (400 MHz,DMSO-d6): δ 7.13 (d,2H,J = 8.2 Hz,Ar),7.04 (dd,3H,J = 15.4,8.2 Hz,Ar),6.98 (d,1H,J = 8.4 Hz,Ar),6.86 (dd,2H,J = 16.0,8.0 Hz,Ar),4.10 (m,2H,CH),2.79 (m,8H,CH2),2.68-2.54 (m,4H,CH2),2.26-1.97 (m,6H,CH2),1.84-1.60 (m,14H,CH2),1.58-1.36 (m,8H,CH2). 13C NMR (101 MHz,DMSO-d6): δ 146.32,146.17,145.82,145.80,138.37,138.26,137.34,137.28,134.99,134.90,133.89,133.81,129.84,129.82,129.40,
129.39,127.75,127.60,119.37,119.23,119.05,118.99,77.18,77.13,76.97,76.94,32.77,30.17,28.83,27.70,27.67,27.58,
23.39,22.49,22.40,22.38,22.33,22.27. 31PNMR (162 MHz,DMSO-d6): δ 145.14,142.85.HRMS (ESI+): calcd. for C46H50NaO6P2 [M + Na]+ 783.2975; found: 767.3002.
A solution of CuTc (0.005 mmol,1.0 mg) and ligand 6a (0.005 mmol,3.7 mg) in Et2O (4 mL) was stirred for 1 h at r.t. under nitrogen. After the solution was cooled to 0 ℃,2- cyclohexenone 7a (0.25 mmol,0.025 mL) was added and the solution was stirred for 10 min at 0 ℃. Then Et2Zn (1.2 mmol,1.2 mL of 1.0 mol/L solution in hexane) was added dropwise using a syringe within 2 min. After 4 h,the reaction was quenched by H2O (2 mL) and 2 mol/L HCl (2 mL),and extracted with ethyl acetate (5 mL × 3). The combined organic layer was washed with saturated NaHCO3 solution,brine,and then dried over anhydrous Na2SO4,filtered,and concentrated in vacuo to obtain the crude product. The conversion and the yield were determined by GC equipped with a SE-30 column (30 m × 0.32 mm ID) using dodecane as an internal standard. The enantiomeric excess was determined by GC with a Chiraldex A-TA column (50 m × 0.25 mm ID),or a CP-Chirasil-Dex CB column (25 m × 0.25 mm ID). The absolute configuration was determined by comparison with authentic samples.
3. Results and discussionThe asymmetric induction ability of the chiral phosphite ligands was thoroughly explored in the Cu-catalyzed ACA of diethylzinc to cyclic enones. Owing to lower sensitivity to air and moisture,Cu(OTf)2 was chosen as the Cu source for the preparation of the optically active catalysts. And 2-cyclohexenone was used as a substrate because this reaction has been performed with a wide range of ligands with several donor groups enabling the direct comparison of the efficiency of various ligand systems. The catalytic system was generated in situ by adding the corresponding ligand to a suspension of catalyst precursor. Results for the application of ligands 6a through 6d are shown in Table 1 (entries 1-4). The use of ligand 6a gave 3-ethylcyclohexanone (8a) in 29% yield and 42% ee (R) (Table 1,entry 1). And ligand 6b,which bears (S)-binaphthyl moieties in comparison with ligand 6a,gave 12% yield and 17% ee (S) (Table 1,entry 2). A 35% yield and 20% ee (R) was gained when using 6c as the ligand (Table 1,entry 3). In contrast,the use of ligand 6d,in which the configuration of the H8- binaphthyl moiety was opposite to that of 6c,gave 17% yield and 15% ee (S) (Table 1,entry 4). It was found that the catalyst prepared in situ from Cu(OTf)2 and ligand 6a was more effective than that from either ligands 6b,6c,or 6d (Table 1,entries 2,3,and 4 vs. entry 1). It is interesting to note that the sense of enantioselectivity was mainly determined by the configuration of the binaphthyl or H8-binaphthyl moiety of ligands 6a through 6d from Table 1.
|
|
Table 1 The Cu-catalyzed enantioselective conjugate addition of diethylzinc to 2-cyclohexenone.a |
A screening of the solvents revealed that the reaction proceeded with significantly higher enantioselectivity in coordinating solvents (Et2O and THF,Table 1,entries 5 and 6) than noncoordinating solvents (toluene and CH2Cl2,Table 1,entries 1 and 7). This result was consistent with the observations of Alexakis et al. [46] and Chan et al. [47] that the asymmetric conjugate addition of diethylzinc to enones gave higher ee values using coordinating solvents when compared to other reaction media. Although THF leads to slightly higher ee values,Et2O was chosen as an appropriate solvent among the solvents examined because of the higher yield.
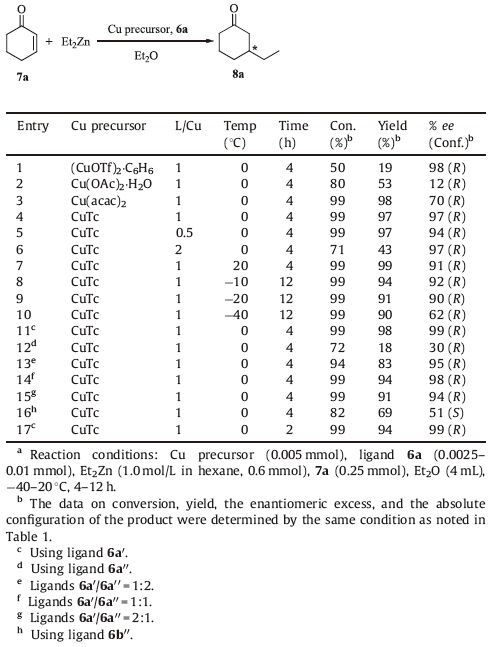
It is well known that the copper precursor plays a crucial role in the high catalytic activity and enantioselectivity of these reactions [48, 49]. So the influence of the copper precursor as well as the copper/ligand ratio on the catalytic performance was examined (Table 2,entries 1-6). In comparison to Cu(OTf)2,(CuOTf)2·C6H6 as a catalytic precursor could dramatically enhance the enantioselectivity in the presence of ligand 6a,but much lower yield was realized (Table 2,entry 1). Interestingly,a better yield (97%) and enantioselectivity (97% ee) were obtained when (CuOTf)2·C6H6 was replaced by CuTc in the presence of ligand 6a (Table 2,entry 4),which suggested that the matched combination of CuTc and ligand 6a under the reaction conditions gave an excellent enantioselectivity and chemical yield of the product 8a. An enhancement in enantioselectivity was obtained when the molar ratio of ligand to CuTc ranged from 0.5/1 to 1/1 (Table 2,entries 4 and 5). Further increase of the ratio of ligand 6a/CuTc resulted in no obvious change of the enantioselectivity,but the yield of the reactions significantly decreased (Table 2,entries 4 and 6) in agreement with our previous discovery [15]. Furthermore,the effect of reaction temperature on the enantioselectivity was investigated and when the temperature was decreased from 20 to 0 ℃,the ee of (R)- enantiomers improved from 91% to 97% (Table 2,entries 4 and 7). With a further decrease of the temperature from 0 to -20 ℃,a lower yield and enantioselectivity were gained (Table 2,entries 7- 9). When the temperature was decreased to -40 ℃,the enantioselectivity was significantly lowered to 62% (Table 2,entry 10). From these results,we can conclude that this novel catalytic system have shown excellent catalytic activity over a wide temperature range (Table 2,entries 7-9).
|
|
Table 2 The Cu-catalyzed enantioselective conjugate addition of diethylzinc to 2-cyclohexenone.a |
The 31P spectrum of the ligand 6a in DMSO-d6 exhibits two singlets with parameters δp 150.75 and 149.31 at a 1.18:1 ratio. In order to verify the authentic catalytic species as racemic diols in the ligands,ligands 6a' and 6a'' derived from enantiopure diols 4' and 1 were prepared and applied in the same reaction,up to 99% ee (R) and 30% ee (R),respectively,were obtained. No significant changes in catalytic performance were observed by changing the ratio of ligands 6a'/6a'' (mol/mol) from 1/2 to 2/1 (Table 2,entries 13-15). From this we can conclude that 6a'/CuTc is the actual catalytic species,however,noting the effect of ligand 6a'' was restrained when mixed ligands were used in the reaction. For ligand 6a' ,the result was similar to that of catalyst 6a/CuTc. In other words,this reaction should proceed effectively by using 6a,which is derived from cheaper racemic starting materials,instead of 6a' as the ligand (Table 2,entries 11 and 12 vs. entry 4). Moreover,we can also conclude that the matching combination of (1R,2R)-trans-1,2-cyclohexanediol and (R)-binaphthyl moieties of ligand 6a' was fundamental to obtaining higher enantioselectivity. Encouraged by this conclusion,we synthesized ligand 6b'' to verify the hypothesis whether there is a matching combination between (1S,2S)-trans-1,2-cyclohexanediol and (S)-binaphthyl moieties of ligand 6b'' ,unfortunately,only 51% ee (S) was received (Table 2,entry 16). Although a standard reaction time of 4 h was chosen,the addition reaction was nearly complete within 2 h (Table 2,entry 17).
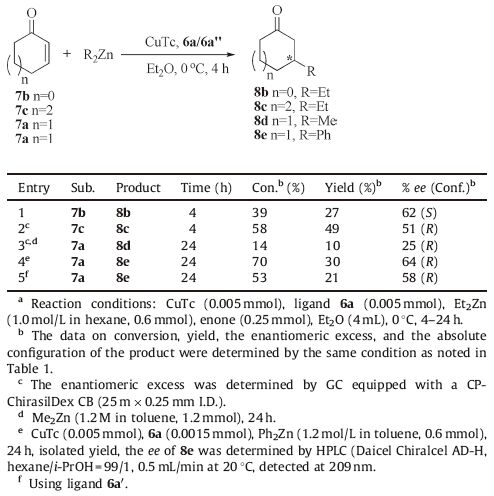
With the optimal reaction conditions in hand,the 1,4-addition of ZnEt2 to 2-cyclopentenone 7b and 2-cycloheptenone 7c in the presence of ligand 6a/CuTc was examined. It can be found that (S)-3-ethylcyclopentanone 8b was obtained in 62% ee,while (R)-3- ethylcycloheptanone 8c was obtained in 51% ee (Table 3,entries 1 and 2). It is interesting to note that the opposite configuration of the product was found when using 2-cyclopentenone instead of 2-cyclohexenone as the substrate. These results indicated a significant dependence of the enantioselectivity on the ring size of the cyclic enones. The Cu-catalyzed asymmetric 1,4-additions of other organozinc reagents,such as ZnMe2 and ZnPh2,to 2- cyclohexenone were also assessed. Unfortunately,when Me2Zn or Ph2Zn was utilized in the reaction,the enantioselectivity decreased to 25% (R) and 64% (R),respectively (Table 3,entries 3 and 4). Similarly,we obtained moderate enantioselectivity for 8e using 6a'/CuTc as the catalyst (Table 3,entry 5).
|
|
Table 3 Cu-catalyzed enantioselective conjugate addition of dialkylzinc to cyclic enones.a |
We have developed a new class of chiral diphosphite ligands derived from racemic and enantiopure diol materials. These ligands were successfully utilized in the copper-catalyzed asymmetric conjugate addition of dialkylzincs to cyclic enones with up to 99% ee. For substrate 2-cyclohexenone,similar catalytic performance was obtained when using ligand 6a instead of ligand 6a' . It was proven that the configuration of products was predominately determined by the configuration of the biaryl moieties of diphosphite ligands. Research concerning the use of these ligands in other transition metal-catalyzed asymmetric reactions is currently underway.
AcknowledgmentsWe are grateful for the financial support of this work by the National Natural Science Foundation of China (Nos. 20773147,21073211,and 21174155).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.10. 009.
| [1] | P.W.N.M. van Leeuwen, P.C.J. Kamer, C. Claver, O. Pàmies, M. Diéguez, Phosphitecontaining ligands for asymmetric catalysis, Chem. Rev. 111 (2011) 2077-2118. |
| [2] | A. Alexakis, J.E. Bäckvall, N. Krause, O. Pàmies, M. Diéguez, Enantioselective copper-catalyzed conjugate addition and allylic substitution reactions, Chem. Rev. 108 (2008) 2796-2823. |
| [3] | S. Toma, J. Csizmadiová, M. Mečiarová, R. Šebesta, Ferrocene phosphane-heteroatom/carbon bidentate ligands in asymmetric catalysis, Dalton Trans. 43 (2014) 16557-16579. |
| [4] | T. Jerphagnon, M.G. Pizzuti, A.J. Minnaard, B.L. Feringa, Recent advances in enantioselective copper-catalyzed 1,4-addition, Chem. Soc. Rev. 38 (2009) 1039-1075. |
| [5] | S.R. Harutyunyan, T. den Hartog, K. Geurts, A.J. Minnaard, B.L. Feringa, Catalytic asymmetric conjugate addition and allylic alkylation with Grignard reagents, Chem. Rev. 108 (2008) 2824-2852. |
| [6] | S. Keithellakpam, W.S. Laitonjam, A simple, efficient and green procedure for Michael addition catalyzed by [C4dabco] OH ionic liquid, Chin. Chem. Lett. 25 (2014) 767-770. |
| [7] | P.M.C. Roth, S.P. Fletcher, Enantioselective copper(I)-phosphoramidite catalyzed addition of alkylzirconium species to acyclic enones, Org. Lett. 17 (2015) 912-915. |
| [8] | J. Westmeier, P. von Zezschwitz, Copper-catalyzed enantioselective 1,4-addition of alkyl groups to N-sulfonyl imines, Chem. Commun. 50 (2014) 15897-15900. |
| [9] | D.Y. Zou, Z.C. Duan, X.P. Hu, Z. Zheng, New bis(1-ferrocenylethyl)amine-derived monodentate phosphoramidite ligands for highly enantioselective copper-catalyzed 1,4-conjugate addition, Tetrahedron: Asymmetry 20 (2009) 235-239. |
| [10] | Y.-L. Chen, R. Fröhlich, D. Hoppe, Copper-catalyzed asymmetric addition of Et2Zn to 2-cyclohexen-1-one and 2-carbamoyloxy-2-cyclohexen-1-one with phosphoramidite, phosphite, and bidentate phosphite-oxazoline ligands, Tetrahedron: Asymmetry 20 (2009) 1144-1149. |
| [11] | F. Monnier, M. Taillefer, Catalytic C-C, C-N, and C-O Ullmann-type coupling reactions, Angew. Chem. Int. Ed. 48 (2009) 6954-6971. |
| [12] | R. Šebesta, M.G. Pizzuti, A.J. Minnaard, B.L. Feringa, Copper-catalyzed enantioselective conjugate addition of organometallic reagents to acyclic dienones, Adv. Synth. Catal. 349 (2007) 1931-1937. |
| [13] | F. Boeda, D. Rix, H. Clavier, C. Crévisy, M. Mauduit, Design and synthesis of new bidentate phosphoramidite ligands for enantioselective copper-catalyzed conjugate addition of diethylzinc to enones, Tetrahedron: Asymmetry 17 (2006) 2726-2729. |
| [14] | W. Zhang, C.J. Wang, W. Gao, X. Zhang, Highly enantioselective copper-catalyzed conjugate addition of diethylzinc to cyclic enones with spirocyclic phosphoramidite ligands, Tetrahedron Lett. 46 (2005) 6087-6090. |
| [15] | A.P. Xing, C.B. Bai, L.L. Wang, Chiral phosphite ligands derived from L-(+)-tartaric acid: synthesis and application in the Cu-catalyzed 1,4-conjugate addition of organozincs to cyclic enones, Tetrahedron 69 (2013) 455-459. |
| [16] | Q.L. Zhao, L.L. Wang, Synthesis of novel chiral bidentatephosphite ligands derived from the pyranoside backbone of monosaccharides and their application in the Cu-catalyzed conjugate addition of dialkylzinc to enones, Tetrahedron: Asymmetry 22 (2011) 1885-1890. |
| [17] | L. Palais, L. Babel, A. Quintard, S. Belot, A. Alexakis, Copper-catalyzed enantioselective 1,4-addition to α,β-unsaturated aldehydes, Org. Lett. 12 (2010) 1988-1991. |
| [18] | Q.L. Zhao, L.L. Wang, A.P. Xing, Synthesis of novel diphosphite ligands derived from D-mannitol and their application in Cu-catalyzed enantioselective conjugate addition of organozinc to enones, Tetrahedron: Asymmetry 21 (2010) 2993-2998. |
| [19] | C. Ladjel, N. Fuchs, J.K. Zhao, G. Bernardinelli, A. Alexakis, New bifunctional substrates for copper-catalyzed asymmetric conjugate addition reactions with trialkylaluminium, Eur. J. Org. Chem. 2009 (2009) 4949-4955. |
| [20] | A. Iuliano, P. Scafato, R. Torchia, Deoxycholic acid-based phosphites as chiral ligands in the enantioselective conjugate addition of dialkylzincs to cyclic enones: preparation of ( )-(R)-muscone, Tetrahedron: Asymmetry 15 (2004) 2533-2538. |
| [21] | L. Liang, M. Yan, Y.M. Li, A.S.C. Chan, Highly enantioselective copper-catalyzed 1,4-conjugate addition of diethylzinc to cyclic enones and α,β-unsaturated lactones, Tetrahedron: Asymmetry 15 (2004) 2575-2578. |
| [22] | S. Guo, Y. Xie, X. Hu, C. Xia, H. Huang, Diastereo-and enantioselective catalytic tandem Michael addition/mannish reaction: access to chiral isoindolinones and azetidines with multiple stereocenters, Angew. Chem. Int. Ed. 49 (2010) 2728-2731. |
| [23] | Y. Xie, H. Huang, W. Mo, et al., Design and synthesis of new chiral pyridine-phosphite ligands for the copper-catalyzed enantioselective conjugate addition of diethylzinc to acyclic enones, Tetrahedron: Asymmetry 20 (2009) 1425-1432. |
| [24] | M.K. Brown, A.H. Hoveyda, Enantioselective total synthesis of clavirolide C. applications of Cu-catalyzed asymmetric conjugate additions and Ru-catalyzed ring-closing metathesis, J. Am. Chem. Soc. 130 (2008) 12904-12906. |
| [25] | Y. Mata, M. Diéguez, O. Pàmies, K. Biswas, S. Woodward, Sugar-phosphite-oxazoline and phosphite-phosphoroamidite ligand libraries for Cu-catalyzed asymmetric 1,4-addition reactions, Tetrahedron: Asymmetry 18 (2007) 1613-1617. |
| [26] | L.T. Liu, M.C. Wang, W.X. Zhao, Y.L. Zhou, X.D. Wang, The ortho effect: coppercatalyzed highly enantioselective 1,4-conjugate addition of diethylzinc to chalcones, Tetrahedron: Asymmetry 17 (2006) 136-141. |
| [27] | Y.N. Yu, M.H. Xu, Chiral phosphite-olefin ligands:application in Rh-catalyzed asymmetric 1,4-addition of arylboronic acids to β-Aryl-α,β-unsaturated sulfonates, Acta Chim. Sin. 72 (2014) 815-819. |
| [28] | J. Kondo, A. Harano, K. Dohi, S. Sakaguchi, C2-symmetric functionalized azolium salt from serine ester for Cu-catalyzed asymmetric conjugate addition reaction, J. Mol. Catal., A: Chem. 395 (2014) 66-71. |
| [29] | F. Ye, Z.J. Zheng, W.H. Deng, et al., Modulation of multifunctional N,O,P ligands for enantioselective copper-catalyzed conjugate addition of diethylzinc and trapping of the zinc enolate, Chem. Asian J. 8 (2013) 2242-2253. |
| [30] | J. Csizmadiová, M. Mečiarová, A. Almássy, B. Horváth, R. Š ebesta, Ferrocene phosphane-carbene ligands in Cu-catalyzed enantioselective 1,4-additions of Grignard reagents to α,β-unsaturated carbonyl compounds, J. Organomet. Chem. 737 (2013) 47-52. |
| [31] | K. Kawamura, H. Fukuzawa, M. Hayashi, Novel N,N,P-tridentate ligands for the highly enantioselective copper-catalyzed 1,4-addition of dialkylzincs to enones, Org. Lett. 10 (2008) 3509-3512. |
| [32] | D.B. Biradar, H.M. Gau, Highly enantioselective conjugate addition of diethylzinc to substituted chalcones catalyzed by Cu(Ⅱ) complexes of a tridentate P,N,O ligand, Tetrahedron: Asymmetry 19 (2008) 733-738. |
| [33] | T. Morimoto, N. Obara, I. Yoshida, K. Tanaka, T. Kan, Copper-catalyzed enantioselective conjugate addition of diethylzinc using axially chiral aminoethyloxyphosphine ligands, Tetrahedron Lett. 48 (2007) 3093-3095. |
| [34] | N. Fuchs, M.đAugustin, M. Humam, et al., Asymmetric conjugate addition of metal alkyl reagents catalyzed by copper complexeswith BINPO: a hemilabile P,O-heterobidentate axially chiral ligand, Tetrahedron: Asymmetry 16 (2005) 3143-3146. |
| [35] | A.P. Duncan, J.L. Leighton, Enantioselective Cu-catalyzed conjugate addition of diethylzinc to acyclic aliphatic enones, Org. Lett. 6 (2004) 4117-4119. |
| [36] | Ö. Dogan, A. Bulut, S. Polat, M. Ali Tecimer, Copper-catalyzed asymmetric conjugate addition of diethylzinc to substituted chalcones using a chiral phosphine ligand, Tetrahedron: Asymmetry 22 (2011) 1601-1604. |
| [37] | Q.L. Zhao, L.L. Wang, F.Y. Kwong, A.S.C. Chan, Cu-catalyzed enantioselective conjugate addition of diethylzinc to cyclic enones with chiral phosphite ligands derived from 1,2:5,6-di-O-cyclohexylidene-D-mannitol, Tetrahedron: Asymmetry 18 (2007) 1899-1905. |
| [38] | R. Bartholomäus, J.A. Irwin, L.W. Shi, et al., Isolation, characterization, and nuclease activity of biologically relevant Chromium(V) complexes with monosaccharides and model diols likely intermediates in chromium-induced cancers, Inorg. Chem. 52 (2013) 4282-4292. |
| [39] | M. Shahjahan Kabir, M. Lorenz, M.L. Van Linn, et al., A very active Cu-catalytic system for the synthesis of aryl, heteroaryl, and vinyl sulfides, J. Org. Chem. 75 (2010) 3626-3643. |
| [40] | X. Cattoën, M.A. Pericàs, Synthesis of highly modular bis(oxazoline) ligands by Suzuki cross-coupling and evaluation as catalytic ligands, Tetrahedron 65 (2009) 8199-8205. |
| [41] | X. Kästele, P. Klüfers, F. Kopp, J. Schuhmacher, M. Vogt, Silicon chelation in aqueous and nonaqueous media: the furanoidic diol approach, Chem. Eur. J. 11 (2005) 6326-6346. |
| [42] | M.D. Groaning, B.J. Rowe, C.D. Spilling, New homochiral cyclic diol ligands for titanium alkoxide catalyzed phosphonylation of aldehydes, Tetrahedron Lett. 39 (1998) 5485-5488. |
| [43] | B. Saha, T.V. RajanBabu, Nickel(0)-catalyzed asymmetric hydrocyanation of 1,3-dienes, Org. Lett. 8 (2006) 4657-4659. |
| [44] | M.J. Bravo, R.M. Ceder, G. Muller, M. Rocamora, New enantiopure P,P-bidentate bis(diamidophosphite) ligands, application in asymmetric Rhodium-catalyzed hydrogenation, Organometallics 32 (2013) 2632-2642. |
| [45] | A. Korostylev, V.I. Tararov, C. Fischer, A. Monsees, A. Börner, Convenient and efficient reduction of 1,1'-binaphthyls to H8-1,10-binaphthyl derivatives with Pd and Ru catalysts on solid support, J. Org. Chem. 69 (2004) 3220-3221. |
| [46] | A. Alexakis, J. Frutos, P. Mangeney, Chiral phosphorus ligands for the asymmetric conjugate addition of organocopper reagents, Tetrahedron: Asymmetry 4 (1993) 2427-2430. |
| [47] | L.L. Wang, Y.M. Li, C.W. Yip, et al., Synthesis of new chiral aryl diphosphite ligands derived from pyranoside backbone of monosacharides and their application in copper-catalyzed asymmetric conjugate addition of diethylzinc to cyclic enones, Adv. Synth. Catal. 346 (2004) 947-953. |
| [48] | A. Alexakis, C. Benhaim, Enantioselective copper-catalysed conjugate addition, Eur. J. Org. Chem. 2002 (2002) 3221-3236. |
| [49] | A. Alexakis, C. Benhaim, S. Rosset, M. Humam, Dramatic improvement of the enantiomeric excess in the asymmetric conjugate addition reaction using new experimental conditions, J. Am. Chem. Soc. 124 (2002) 5262-5263. |
 2016, Vol.27
2016, Vol.27